Key Facts
- •
Osteoporosis, osteomalacia, osteosclerosis, hyperostosis, and osteonecrosis may be related to drug effects.
- •
Methotrexate osteopathy is characterized by bone pain, osteoporosis, and insufficiency fractures.
- •
Antiepileptic drugs may cause rickets, osteomalacia, osteoporosis, and increased risk for fracture, and phenytoin can cause calvarial thickening.
- •
Retinoids may produce birth defects, hyperostosis, ligament calcification, or ligament ossification.
- •
Fluorosis results in characteristic skeletal changes, including osteosclerosis and calcification or ossification of tendons and ligaments.
- •
Fluoroquinolones may produce tendinopathy or tendon tears, especially in patients with other predisposing conditions.
- •
Demyelinating lesions in the central nervous system may occur in patients receiving antitumor necrosis factor alpha drugs.
- •
Infection may complicate immunosuppressive or anti-TNF alpha drugs.
In this chapter, musculoskeletal and teratogenic side effects of certain drugs and chemical substances are reviewed. Some of the medications taken for the treatment of arthritis and skin and epileptic diseases, as well as corticosteroids, anticoagulants, and antineoplastic drugs, can profoundly affect the skeleton. Osteoporosis, osteomalacia, osteosclerosis, and hyperostosis may result.
TERATOGENIC DRUGS
A teratogen is an agent that can disturb the development of the embryo or fetus, resulting in spontaneous abortion, congenital malformations, intrauterine growth retardation, mental retardation, carcinogenesis, or mutagenesis. Known teratogens include radiation, maternal infections, chemicals, and drugs. Among possible teratogens, drugs account for approximately 1% of all congenital malformations of known etiology. All teratogenic drugs generally produce a specific pattern or single malformation during a sensitive period of gestation with a dose-dependent effect.
Teratogenic drugs generally produce a specific pattern of abnormalities or a single malformation during a sensitive period of gestation with a dose-dependent effect.
The American Food and Drug Administration (FDA) instituted a rating system for drugs marketed after 1980 based on their safety during pregnancy. Five pharmaceutical categories have been elaborated: A, B, C, D, and X. Drugs under Category A are the safest drugs in which no fetal risks have been demonstrated during controlled human studies, while those under Category X present proven teratogenicity that clearly outweighs their benefits. Category D drugs have demonstrated risks to the human fetus, but in serious diseases or life-threatening situations their benefits outweigh these risks.
Retinoids
The term retinoids includes all compounds, synthetic and natural, that possess vitamin A activity. Isotretinoin, etretinate, and acitretin are potent teratogens. The birth defects characteristically induced by oral retinoids known as retinoic acid embryopathy include abnormalities involving central nervous system, cardiovascular system, craniofacial structures, thymus, and skeletal system. Retinoid effects on neural crest cells during the fourth week after fertilization may be responsible for many of the observed malformations.
The most common reported craniofacial and skeletal malformations include microcephaly, cleft plate, micrognathia, abnormalities of the external ears (anotia, microtia, rudimentary ears), and abnormal or absent auditory canals. Affected children may also have a depressed midface, large occiput, and narrow frontal bone. Limb reduction and duplication have also been reported. The clinical expression of retinoic acid embryopathy may vary with type of the retinoid; etretinate is more likely to induce acral skeletal malformations and less likely to induce cardiac malformations. Cardiac malformations include atrial and ventricular septal defects, overriding aorta, interrupted or hypoplastic arch, and subclavian arteries. Central nervous system abnormalities range from retinal or optic nerve abnormalities to hydrocephalus and cognitive and behavioral changes ( Table 15-1 ).
| DRUGS (FDA Category) | Maternal Condition | Musculoskeletal Anomalies | Other Anomalies |
|---|---|---|---|
| Thalidomide (X) | Insomnia, oropharyngeal and esophageal ulcers associated with AIDS, immunopathologic disease, multiple myeloma, graft-versus-host disease, leprosy | Limb reduction | Cardiac defects, renal and gastrointestinal anomalies, deafness, mental retardation, autism |
| Retinoids (X) | Dermatologic disease | Facial dysmorphia | CNS, ear and cardiac malformations |
| Coumarin derivatives dicumarol, warfarin | Thromboembolic disorders | Stippled epiphyses | CNS anomalies, intracranial hemorrhage |
| Anticonvulsants (D) carbamazepine, clonazepam, ethosuximide, phenobarbital, phenytoin, primidone, trimethadione, valproic acid | Epilepsy | Digital hypoplasia, facial dysmorphia | CNS (neural tube defects), cardiac and genitourinary anomalies |
| Folic acid antagonists (D) methotrexate | Cancer, rheumatic diseases | Large fontanelles, abnormal head shape, craniosynostosis, skeletal defects |
Anticonvulsants
The overall risk of congenital anomalies among the infants of epileptic mothers treated with antiepileptic drugs during pregnancy is 2 to 3 times higher than the “baseline” risk of every pregnancy, which has been estimated to be between 3% and 5%.
The risk of fetal malformation is increased up to 15% with polytherapy. The combination of valproic acid, carbamazepine, and phenytoin or phenobarbital seems to have the highest risk.
The diphenylhydantoin syndrome occurs in 5% to 10% of babies born to a mother under therapy with the drug. The syndrome includes prenatal onset of growth deficiency, large anterior fontanelle, metopic ridging, ocular hypertelorism and depressed nasal bridge, cleft lip with or without cleft palate, distal phalangeal hypoplasia, digitalized thumb and nail hypoplasia, and cardiac and genitourinary anomalies. Valproic acid has been associated with neural tube defects.
Warfarin
The oral anticoagulant warfarin has been recognized as a human teratogen for many years. Warfarin embryopathy is seen in approximately 10% of fetuses with first-trimester exposure to coumarin. This distinct pattern of anomalies includes nasal hypoplasia, depressed nasal bridge, and “stippling” of epiphyses of spine, proximal femora, and tarsal and carpal bones, which are visible radiographically. X-linked recessive chondrodysplasia punctata (CDPX) and warfarin embryopathy share the same phenotype. A different pattern of anomalies with CNS defects is seen with second-trimester and third-trimester exposure to coumarin, possibly secondary to fetal hemorrhage.
Folic Acid Antagonists
Aminopterin and its methyl derivative, methotrexate, are folic acid analogs with antagonistic effects. Methotrexate is currently used in high doses as an antineoplastic agent and in low doses for a variety of rheumatic conditions. These agents have been used to induce abortion in early pregnancy, but their use later in gestation results in prenatal growth deficiency, abnormal skull ossification, ocular hypertelorism, supraorbital ridge hypoplasia, malformed ears, and micrognathia. The skeletal abnormalities may also include talipes equinovarus, short extremities, syndactyly, absent digits, and multiple anomalous ribs.
Thalidomide
Thalidomide was recognized as a human teratogen in the early 1960s when an unusually large number of infants with severe limb defects and other anomalies were noted in Europe in association with the maternal use of thalidomide. It was used as a sleeping pill and to treat morning sickness during pregnancy. It is estimated that more than 10,000 pregnancies were exposed before the drug was withdrawn from the market worldwide.
The risk of teratogenicity is highest between the 34th and 50th days of the pregnancy. Characteristically, thalidomide exposure produces reduction deformities of the limbs, such as dysplasia of the thumbs and radial hemimelia, phocomelia, or complete four limb amelia. Other defects of thalidomide embryopathy include hypoplasia or aplasia of the external ear canal, congenital heart defects, gastrointestinal atresia, and renal malformations.
Originally not approved for use in the United States, in 1998 the FDA approved the use of thalidomide for treatment of erythema nodosum leprosum. Currently, its other potential uses in the treatment of AIDs, autoimmune disorders, and multiple myeloma have been suggested, leading once again to concern about thalidomide-induced birth defects.
DRUGS ASSOCIATED WITH OSTEOPOROSIS AND OSTEOMALACIA
Corticosteroids
Possible effects of local injection of corticosteroids are discussed in the chapter on joint injections (see Chapter 5 ).
Corticosteroid-induced osteoporosis is multifactorial and dose dependent. In a metaanalysis by van Staa et al, cumulative dose has been noted to be strongly correlated with loss of bone mineral density and daily dose to be strongly correlated with the risk of fracture. The risk of fracture was found to increase rapidly after the start of oral corticosteroid therapy (within 3 to 6 months) and decrease after stopping therapy. In another study by van Staa et al, a dose dependence of fracture risk was observed; with a standardized daily dose of less than 2.5 mg prednisolone, hip fracture risk was 0.99 (0.82-1.20) relative to control, rising to 1.77 (1.55-2.02) at daily doses of 2.5 to 7.5 mg, and 2.27 (1.94-2.66) at doses of 7.5 mg or greater.
Manelli and Giustina note corticosteroid treatment to be associated with increased bone resorption, inhibition of bone formation, decreased intestinal calcium absorption, changes in vitamin D metabolism, and marked hypercalciuria, with variable changes in plasma PTH levels and inhibition of the gonadotropic and somatotropic axis.
Comparison of iliac crest samples from subjects with idiopathic osteoporosis to those with corticosteroid-induced osteoporosis has shown differences in bone microstructure. Aaron et al. showed that loss of trabecular bone volume is common to both groups, but there is a difference in the distribution of the remaining bony tissue and indices of remodeling. A decrease in trabecular number accompanied by a relative increase in resorption characterized primary osteoporosis, whereas a decline in trabecular width associated with depressed formation was the predominant feature in the secondary disease. Investigational micro MRI and CT studies have been used in vivo to better understand the microarchitectural changes in trabecula in osteoporosis including corticosteroid induced osteoporosis.
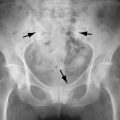
Imaging of osteoporosis is discussed in Chapter 31 . Radiographic features of corticosteroid-induced osteoporosis are usually indistinguishable from those of osteoporosis. Insufficiency fractures may result, involving thoracic and lumbar vertebrae, the sacrum, the long bones, and the calcaneus. Generally in patients with acute low back pain (less than 6 weeks in duration) symptoms are self-limited and not considered an indication for imaging. However, osteoporosis or a history of prolonged corticosteroid use are “red flags” that should prompt imaging. Radiographs are the first study suggested. Sclerosis along the compressed vertebral margins is a finding that may be prominent following corticosteroid-related compression fracture (Barbara Weissman, personal communication) ( Figure 15-1 ).

In patients with painful osteoporotic compression fractures treated with vertebroplasty, subsequent fracture has been reported to be more likely to occur in corticosteroid-treated patients than in those with primary osteoporosis. Hiwatashi and Westesson found the incidence of subsequent vertebral compression fractures after vertebroplasty in patients on long-term corticosteroid therapy to be 69% (11/16), compared with 23% (9/39) in those who were not on corticosteroid therapy.
Patients on corticosteroids undergoing vertebroplasty appear more likely to develop subsequent vertebral fractures than are patients with primary osteoporosis.
Methotrexate
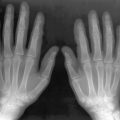
Methotrexate (MTX) is a folate antagonist commonly used for the treatment of various childhood malignancies and in low doses to control rheumatoid arthritis and psoriasis. Its hematologic, hepatic, and pulmonary toxicities are well recognized. The use of high-dose MTX therapy in pediatric oncology, especially during treatment of acute lymphatic leukemia and osteosarcoma, has been associated with an osteopathy, which is characterized by severe bone pain, osteoporosis, and insufficiency fractures. The radiographic changes of MTX osteopathy resemble those of scurvy with severe osteopenia, dense zones of provisional calcification, multiple transverse metaphyseal bands, and metaphyseal fractures involving multiple bones, more frequently in lower extremities ( Figure 15-2 ). The bone pain usually improves within three to four weeks after discontinuing the drug, although the radiographic changes take about four months to resolve. Delayed healing of fractures has also been reported, with bone union not occurring until the MTX has been discontinued.


Methotrexate osteopathy can produce changes on radiographs that resemble those of scurvy with severe osteopenia, dense zones of provisional calcification, multiple transverse metaphyseal bands, and metaphyseal fractures.
Detrimental effects of MTX on the skeleton in patients with rheumatic diseases is more controversial because most patients using MTX have other risk factors for fractures. There have been sporadic case reports of fragility fractures seen in adult patients on low-dose MTX for rheumatoid or psoriatic arthritis. Uehara et al have shown in vitro that methotrexate impairs bone formation dose dependently by inhibiting the differentiation of osteoblast precursors. The proliferation and further maturation of cells of the osteoblast lineage are not affected by treatment with MTX. Recent studies have shown no definite adverse effect of long-term, low dose MTX therapy on bone mineral density (BMD) or bone turn over.
Antiepileptic Drugs
The antiepileptic drugs (AED) can adversely affect the bones in patients of all ages. Chronic AED therapy can cause rickets, osteomalacia, and osteoporosis with increased risk for fracture.
Several theories have been proposed to explain the link between AEDs and metabolic bone changes. These theories include increased catabolism of vitamin D, impairment of calcium absorption, alteration of bone resorption and formation, abnormal PTH release or cellular responsiveness to PTH, and abnormalities in calcitonin or vitamin K. Antiepileptic drugs (phenytoin, phenobarbital, and carbamazepine) that lead to induction of the cytochrome P-450 enzyme system have been most commonly associated with bone abnormalities. Valproate, a newer AED that inhibits the cytochrome P-450 enzyme system, also appears to affect bone adversely. The AEDs that induce cytochrome P-450 enzymes may cause increased conversion of vitamin D to inactive metabolites in the liver, reducing levels of vitamin D. Reduced levels of biologically active vitamin D lead to decreased absorption of calcium from the gut, resulting in hypocalcemia and an increase in circulating parathormone.
Early reports described a high incidence of rickets and osteomalacia in patients treated with AEDs. However, these reports primarily involved institutionalized patients in whom low dietary calcium and vitamin D intake and reduced exercise level and sunlight exposure probably influenced outcomes. Radiologic evidence of osteomalacia and rickets is rarely found in ambulatory patients ; when present, radiographic changes are indistinguishable from those of rickets or osteomalacia resulting from other causes. Radiologic findings of rickets appear as widening of growth plate, indistinctness of zones of provisional calcification, widening and cupping of the metaphysis, and deformities of bones. Osteomalacic changes of the bone are seen on radiographs as a decrease in radiographic bone density, coarsened trabecular pattern, and looser zones. Radiologic findings of secondary hyperparathyroidism may accompany these findings.
Rickets/osteomalacia or osteoporosis can be a side effect of antiepileptic drug treatment.
Children with convulsions and tuberous sclerosis appear to be particularly vulnerable to developing AED-induced rickets. The diuretic acetazolamide (Diamox), which is a carbonic anhydrase inhibitor, produces a renal tubular acidosis with increased excretion of calcium and phosphorus and can accentuate AED-induced rickets or osteomalacia.
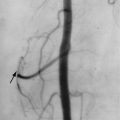
Long-term treatment with AEDs is a recognized factor that can contribute to the development of osteoporosis. Several studies using dual x-ray absorptiometry (DXA) in patients receiving AEDs have shown significantly reduced BMD, both in adults and children. AED use has been reported to increase the fracture risk, independent from the increased fracture rate described in epileptic patients ( Figure 15-3 ).

In addition, phenytoin (Dilantin) has been associated with calvarial thickening and enlargement of the heel pad, similar to the changes occurring in acromegaly.
Deferoxamine
Deferoxamine is an iron-chelating agent used to remove excess iron during the treatment of patients with transfusion-dependent anemias such as beta-thalassemia major. Iron overload caused by hypertransfusion may result in toxicity and dysfunction of the heart, liver, and endocrine organs. Parenteral chelation therapy with deferoxamine prolongs the life expectancy in these patients; however, deferoxamine therapy also has its own risks, causing sensorineural ototoxicity, ocular toxicity, growth retardation, and bone dysplasia. These undesirable effects of deferoxamine can be largely avoided if optimal dose and timing of the therapy is adjusted.
In 1988, de Virgiliis et al. described radiologic abnormalities similar to those of rickets in the metaphyses of long bones in 70% of thalassemic patients undergoing chelation therapy. Growth retardation and metaphyseal abnormalities were noted to occur frequently in patients in whom chelation was started before the age of 3 years. The mechanism by which deferoxamine affects bone is not entirely understood. Chelation of zinc and the antiproliferative effect of deferoxamine may be the underlying pathophysiologic mechanisms.
The radiologic changes seen in metaphyses of long bones are most commonly detected in the distal ends of the ulna, radius, femur, and proximal tibia. These include peripheral deficiency of bone at the metaphysis, asymmetric widening of the growth plate, irregular metaphyseal sclerosis, and lucencies with sclerotic margins.
Radiographic findings following deferoxamine treatment include peripheral deficiency of bone at the metaphysis, asymmetric widening of growth plate, irregular metaphyseal sclerosis, and lucencies with sclerotic margins that are most prominent at the wrists and knees.
The thoracic and lumbar vertebrae may show flattening with loss of height. Olivieri et al. demonstrated significant decline in height percentile in patients who started on deferoxamine prior to age 2 years.
MRI findings of deferoxamine-induced bone dysplasia in the distal femur and patella in thalassemic patients have been described by Chan and his colleagues. They detected blurring of the physeal-metaphyseal junction, hyperintense areas in the distal metaphysis, and physeal widening. Physeal widening and distal metaphyseal hyperintensities were all more pronounced peripherally. Linear or irregular low-signal–intensity foci somewhat similar to the MR appearance of immature bone infarcts were also described in the metaphyses and epiphyses.
Vitamin D
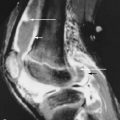
Vitamin D intoxication in children is usually accidental but may develop during the treatment of rickets. Clinical manifestations include anorexia, weakness, hypotonia, constipation, and lethargy. The level of serum calcium becomes elevated. Metaphyseal bands of sclerosis, reflecting heavy calcification of the proliferating cartilage, can be seen in the tubular bones ( Figure 15-4 ).

In adults, hypervitaminosis D can be seen in patients with rheumatoid arthritis, gout, or Paget’s disease who are treated with excessive doses of vitamin D. It can lead to generalized osteoporosis and metastatic soft tissue calcification in the periarticular soft tissues, tendon sheaths, joint capsules, and synovial bursae.
Other Drugs Associated with Osteoporosis and Osteomalacia
Heparin
Heparin when administered in large doses (greater than 15,000 units/day) can induce osteoporosis. Typical radiographic findings are osteopenia and multiple rib and vertebral compression fractures.
Gonadotrophin-Releasing Hormone
Gonadotrophin-releasing hormone (Gn-RH) and its analogues as androgen deprivation therapy are prescribed in patients with locally advanced or metastatic prostate cancer. Treatment with GnRH increases bone turnover, decreases BMD, and increases fracture risk.
Aromatase Inhibitors
The aromatase inhibitors (AI) anastrozole, letrozole, and exemestane are used for the adjuvant treatment of estrogen-receptor–positive early and advanced breast cancer in postmenopausal patients.
Aromatase inhibitor therapy increases the risk of osteoporosis and associated bone fractures.
Ifosfamide
Ifosfamide is a derivative of cyclophosphamide, commonly used in the treatment of sarcomas and other solid tumors. One potential toxicity of its use is renal tubular damage, which can lead to skeletal abnormalities, rickets in children, and osteomalacia in adults. Hypophosphatemic rickets occurs in 5% to 18% of children treated with ifosfamide and may be the first manifestation of the underlying renal damage.
Bisphosphonates
Growth Changes
The effects of bisphosphonate therapy in growing skeleton have been described by van Persijn van Meerten et al. in 9 pediatric patients. They observed radiographic findings of band-like metaphyseal sclerosis, bone-within-bone appearance, and metaphyseal undertubulation, presumably due to inhibition of osteoclastic activity and relative increase in bone formation. It has been reported that metaphyseal sclerosis is reversible after discontinuation of medication before closure of the growth plates and in patients receiving continued medication after closure of the growth plates.
Two complications of bisphosphonate treatment have received recent attention.
(1) Osteonecrosis of the mandible: Bisphosphonates are used in the treatment of postmenopausal osteoporosis, Paget’s disease, steroid induced osteoporosis, multiple myeloma, and other conditions. Osteonecrosis of the mandible is a rare condition that may occur in patients taking nitrogen-containing bisphosphonates (e.g., alendronate, risedronate, pamidronate, zoledronic acid, and ibandronate ). Conditions that predispose to the development of this complication include cancer and anticancer treatment, intravenous administration of bisphosphonates, dental extractions and surgery involving the mandible or maxilla, duration of treatment, glucocorticoids, comorbidities such as malignancy, smoking and alcohol, and pre-existing dental or periodontal disease. The incidence of osteonecrosis in patients taking oral preparations is estimated at between 1 in 10,000 and < 1 in 100,00 patient treatment years. In patients with cancer receiving high doses of intravenous bisphosphonates, the incidence is higher, ranging from 1 to 10 per 100 patients.
Osteonecrosis of the mandible is a rare condition that may occur in patients taking nitrogen-containing bisphosphonates and may lead to painful areas of exposed bone that are susceptible to infection.
The mandible is affected more often than the maxilla. Areas of exposed bone are susceptible to infection and are slow to heal or do not heal. Pain is the predominant manifestation, but loose teeth or a draining fistula may occur. There is no curative treatment. Several articles have reviewed the features of this condition and provide recommendations for clinical management before and after bisphosphonate therapy.
Radiographs remain the initial imaging modality although they are not early detectors of the condition. Sclerosis, with mottling and bone fragmentation and sequestration and persistent extraction sockets may be seen. CT can better define areas of sclerosis or bone destruction (lysis) due to secondary infection. Periosteal reaction may be visible. Contrast enhanced MRI may be most useful, as the area of ischemia will not enhance. Unenhanced MRI is less valuable but may show loss of normal marrow signal on T1-weighted images and cortical fragmentation. FDG-PET scanning has shown increased standardized uptake value (SUVmax) in areas of osteonecrosis.
(2) Atypical fractures of the femoral shaft: Subtrochanteric or proximal diaphyseal femoral fractures have been seen after minimal trauma (such as a fall from a standing height or less) in postmenopausal woman treated with alendronate. The pattern of these fractures on radiographs was described as “unique”. Thus 10 of the 15 patients demonstrated a simple transverse or oblique fracture with beaking of the cortex and diffuse cortical thickening of the proximal femoral shaft. Interestingly, cortical thickening was also seen in the contralateral femur. Kwek et al. noted that these fractures most likely represent completion of a prior stress fracture. Most patients have had prodromal thigh pain, vague discomfort, or weakness. These authors therefore suggest that patients on bisphosphonates who have thigh pain should have radiographs of the femur and that any patient with a complete fracture should undergo radiographic examination of the opposite femur to detect stress changes or incomplete fractures. MRI, CT, or bone scanning may be useful in this assessment as well. The cause of these fractures remains incompletely understood.
Subtrochanteric or proximal femoral shaft fractures may occur with minimal trauma in patients taking bisphosphonates.
Other Effects
Disodium etidronate is a bisphosphonate used in low doses for treatment of Paget’s disease. The major complication of etidronate treatment is the inhibition of normal skeletal mineralization, leading to a clinical and histologic picture of “focal” osteomalacia. Spontaneous fractures of uninvolved bones of patients with Paget’s disease during treatment with disodium etidronate have been described.
DRUGS ASSOCIATED WITH OSTEOSCLEROSIS AND PROLIFERATIVE CHANGES
Vitamin A
Vitamin A provided by the diet is found in two forms: (1) preformed vitamin A, found naturally only in animal products; and (2) carotenoid vitamin A precursors (provitamin A), found primarily in foods of plant origin. Hypercarotenemia has not been shown to have any adverse systemic effects. On the other hand, excessive intake of preformed vitamin A is toxic. The clinical presentation and radiologic findings of vitamin A toxicity are related to the acute or chronic nature of vitamin abuse and the patient’s age.
Acute hypervitaminosis A may occur after ingestion of 500,000 IU (100 times the recommended dietary allowance [RDA]) or more in adults (proportionately smaller doses in children) over a short period of time. The most common symptoms of acute vitamin A toxicity include nausea, vomiting, headache, and irritability. In children, bulging of fontanelles and widening of the sutures due to acute transient hydrocephalus can be seen and usually resolve within 36 to 48 hours on cessation of overdosing.
Chronic hypervitaminosis A in humans has been reported after recurrent intakes of retinol in amounts > 10 times the RDA in adults. Chronic hypervitaminosis A is more common than acute hypervitaminosis and often goes unrecognized. Bone pain, eczema, hair loss, anorexia, pseudotumor cerebri, liver disease, and psychiatric complaints have been described with chronic hypervitaminosis A. Children are particularly sensitive to vitamin A, with daily intakes of 1500 IU/kg body weight reportedly leading to toxicity. Skeletal changes observed in children exposed to high doses of chronic vitamin A are cortical thickening of the tubular bones, typically in the ulnae and metatarsal bones, cupping and splaying of metaphyses, and irregularity and narrowing of the growth plates and premature fusion of epiphyseal ossification centers ( Figure 15-5 ). The irreversible damage to epiphyseal cartilage can result in short stature, leg length discrepancy, and flexion contractures.


Bone changes of chronic hypervitaminosis A in children include cupping and splaying of metaphyses, irregularity and narrowing of the growth plates, premature fusion of epiphyseal ossification centers, and cortical thickening of the tubular bones.
Bone changes in vitamin A poisoning are rare in adults, but proliferative enthesopathies involving both long bones and the flaval ligaments of the spine have been noted.
In recent years, studies conducted in Scandinavia and the United States found associations between preformed vitamin A intake and hip fracture or osteoporosis. These studies suggest that intakes as low as twice the current RDA can increase the risk for osteoporosis; however, further research is needed to clarify whether subclinical toxicity of vitamin A exists or if other synergistic nutritional or nonnutritional factors enhance bone fragility.
Retinoids
The term retinoids includes all compounds, synthetic and natural, that possess vitamin A activity.
Today three generations of retinoids have been developed. Isotretinoin is a first generation drug that was confirmed to be highly effective against nodular cystic acne and gained approval from the Food and Drug Administration (FDA) in 1982 for treatment of that condition. The second generation retinoids, etretinate and acitretin, also known as aromatic retinoids, are most effective in treating psoriasis and keratinizing disorders. The third generation retinoids include tazarotene and adapalene, which are FDA-approved topical agents for psoriasis and acne, respectively.
Musculoskeletal Side Effects
The main adverse skeletal effect of oral retinoids is skeletal hyperostosis, manifested as vertebral osteophytes, bony bridges, and ossification of the anterior longitudinal ligament and extraspinal ossifications of tendons and ligaments ( Figures 15-6 to 15-8 ). These findings are reminiscent of those seen in diffuse idiopathic skeletal sclerosis (DISH). Spinal alterations predominate in the cervical region. Etretinate and acitretin may selectively target the peripheral entheses most notably at the calcaneus, pelvis, and knee. A few cases of ossification of the interosseous membrane at the forearm have been described in patients on etretinate. The extent of radiologic ossification increases with the type, dosage, and duration of the retinoid therapy. The average time for development of radiologic abnormalities is usually 24 to 36 months with etretinate and 10 months with isotretinoin. Hyperostoses are irreversible, but they are generally asymptomatic.






Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree