Imaging of congenital heart disease is a complex and challenging topic that until recently was almost exclusively the domain of pediatric radiologists and cardiologists. With advances in surgical and percutaneous techniques since the 1990s, most patients with congenital heart disease now survive into adulthood and are imaged with increasing frequency. Adults with corrected congenital heart disease often come to attention decades after initial surgical repair when they become symptomatic from long-term complications arising from failure of their postoperative anatomy or from secondary complications. Most of these patients underwent surgical procedures long before the development of electronic medical record keeping, and they are often imaged at different institutions with little information available on the underlying lesion or surgical repair. The role of the imager in these situations is to recognize the lesion and the corrective measures, as well as the complications that bring the patient to clinical attention. Cross-sectional imaging now complements, and has sometimes come to replace, echocardiography and angiography because of technical advances in CT and magnetic resonance imaging (MRI), as well as the widespread availability of these modalities both in the emergency and outpatient settings. Therefore, every radiologist must be familiar with the cross-sectional imaging appearance of the most common congenital heart lesions, the basic operations used to correct them, and the long-term complications of surgical correction.
Providing a detailed account of every congenital heart defect and every conceivable corrective surgical procedure or complication is beyond the scope of this chapter. Instead, the focus is on the most common heart defects survived into adulthood and the most common operations, with the hope of giving the reader a basic framework for pattern recognition and problem solving when faced with an adult patient presenting with unknown, corrected congenital heart disease.
Techniques
CT and MRI have few standard protocols for cardiac imaging of congenital heart disease. The modality and technique must be tailored to the patient and the clinical question. CT angiography (CTA) before and after the administration of intravenous nonionic contrast material with thin collimation on a 64-slice or newer-generation scanner is the preferred CT method of evaluation. Low-dose precontrast imaging may be important because it allows identification of calcified or high-density conduits and repair changes and lessens the risk of confusing these normal changes with postoperative complications such as vascular leaks. In most cases, automated bolus tracking or test bolus techniques to optimize contrast opacification to the aorta or vascular bed of interest are used, although this approach can certainly be altered depending on the congenital disease and the desired information. Splitting the contrast bolus with two different rates of injection, similar to performing triple rule out CTA, can be done to obtain adequate opacification of both the pulmonary and systemic arterial systems. One must be prepared to obtain delayed phase imaging because altered anatomy, such as secondary to Fontan procedures or cavopulmonary shunts, may not opacify on the initial phase as a result of upper extremity contrast injection. A fixed delay may be required when the clinical question centers on the right heart or superior vena cava (SVC). Generally, a 15- to 20-second delay allows for the evaluation of these structures and can be quite useful when the clinical issue concerns a Glenn shunt or the superior limb of a baffle.
Because coronary artery anomalies are common in many types of congenital heart disease, electrocardiogram (ECG) gating can also be performed for dedicated coronary analysis. Although dose reduction techniques are making inroads to lessen the issue of radiation exposure even with retrospective gating, coronary anomalies in origin and course are generally readily apparent on thin collimation examinations in the absence of gating. As a rule, multiphase imaging of the thorax is preferred to the added radiation of gating. Gated studies are usually used as problem-solving techniques or when ventricular parameters are required. If gating is to be performed, care must be taken with the use of beta-blockers in patients who are in right-sided heart failure because the use of these drugs can exacerbate heart failure. CTA also provides excellent multiplanar and three-dimensional postprocessing capability, as well as potentially functional information (if retrospective ECG-gating has been used). The advantages of CT compared with MRI are faster imaging time and more widespread availability.
A basic cardiac MRI protocol often includes noncine fast black-blood scout images in axial, sagittal, and coronal views throughout the chest (single shot half-Fourier fast spin echo sequences) to evaluate mediastinal and great vessel anatomy. These images are often followed by transaxial bright-blood images, which are usually gated, noncine single shot steady-state free precession (SSFP) images. Intravenous gadolinium-based contrast agents are often not required for congenital heart disease analysis, although angiographic postcontrast sequences can help clarify vascular anomalies or stenoses when information is unclear on noncontrast sequences. Breath-hold noncontrast SSFP cine images are obtained along both the short and long axis of the heart to assess the anatomy and function of the cardiac chambers. On these cine images, laminar flow appears as high signal because of flow-related enhancement. A low-signal jet appears on the high-signal background in areas of turbulence as a result of dephasing of protons in areas of nonlaminar flow. Close attention must therefore be paid to dephasing artifact, especially on the cine images, because this artifact occurs in areas of vascular or valvular stenosis, in regurgitation, and across shunt defects. If mural thrombus in a surgical conduit or in the left atrial appendage is of clinical concern, a spoiled gradient echo or fast low-angle shot sequence may be used. This sequence does not have the chemical shift artifact inherent in the SSFP and is less likely to obscure subtle thrombus.
In patients with an intracardiac or extracardiac shunt, cross-sectional flow quantification of the proximal aorta and pulmonary artery by using a phase-encoded inversion recovery sequence can be performed to calculate the ratio of pulmonic flow to systemic flow (Qp/Qs). The same sequence when positioned perpendicular to a dephasing jet can be used to calculate the maximum velocity of flow across areas of stenosis, with the gradient across the stenotic segment calculated using the modified Bernoulli equation [gradient in mm Hg = 4 × (peak velocity in m/second) 2 ]. When the slice position is placed in cross section above the aortic or pulmonic valve, a regurgitant volume and fraction can be calculated. Limitations of MRI include claustrophobia and the inability to image patients with non–MRI-compatible cardiac pacemakers, which represent most currently available devices.
Bicuspid Aortic Valve
Bicuspid aortic valve, the most common type of congenital heart disease, with an incidence of 0.9% to 2% in the general population, is the result of incomplete separation of the valve leaflets during embryogenesis. Morphologically, the incompletely separated leaflet usually contains a midline cleft or raphe, which is readily detected by echocardiography and cross-sectional imaging. In the most common form of this condition, the right and left leaflets comprise the “fused” leaflet, and the noncoronary leaflet is normal. This anomaly has a male predominance, with a male-to-female ratio of 4:1. Patients with a bicuspid valve are at risk of developing infectious endocarditis and require antibiotic prophylaxis before invasive procedures. Coarctation of the aorta and interrupted aortic arch are frequently associated with a bicuspid aortic valve. Because of this association, the diagnosis of any of these conditions warrants interrogation for the others on initial imaging.
Although patients may be initially asymptomatic and a few may never become symptomatic, the natural history of bicuspid valve is progressive, premature calcification and degeneration of the valve leaflets resulting in aortic stenosis and, sometimes, concomitant regurgitation. Because of coexisting aortopathy, dilation of the aortic root or ascending aorta may be seen, the presence and degree of which are independent of the patient’s age or the degree of valve stenosis. If aortic regurgitation is present, left ventricular dilation may develop, and aortic dilation can accelerate. Patients with a bicuspid aortic valve are predisposed to aortic dissection, also a function of the concomitant aortopathy, independent of the functional impairment of the valve. Therefore, in patients less than 40 years old who present with aortic dissection, bicuspid aortopathy, as well as heritable diseases such as Marfan syndrome, should be considered.
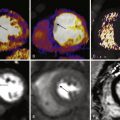
Echocardiography is the primary imaging modality in the evaluation of the aortic valve because of its ease of performance, widespread availability, relatively low cost, excellent spatial and temporal resolution, ability to estimate gradients and valve areas, and lack of need for nephrotoxic contrast agents and radiation. Because many of these patients are regularly followed up, the benefits of echocardiography make it an ideal choice for repetitive imaging, especially when patients are young. However, the use of CT and MRI for preoperative and postoperative imaging of a bicuspid aortic valve is becoming more common, usually to evaluate and follow aortic size. Both CT and MRI can provide high-resolution images of the aortic valve en face to characterize the morphology of the valve and allow for valve planimetry. CT is superior at demonstrating calcification, which is common in stenotic valves, as well as allowing for simultaneous evaluation of the coronary arteries and complete thoracic aortic assessment before surgical intervention ( Fig. 20-1 , A to D ).

On MRI, phase contrast flow quantification sequences allow pressure gradient estimation from peak velocities across the aortic valve. After an oblique coronal view of the aortic root is obtained with a cine SSFP sequence, the flow quantification series is run with the imaging plane perpendicular to the dephasing jet of stenosis that occurs above the aortic valve and contains the maximum velocities (see Fig. 20-1 , E and F ). When the series is run perpendicular to the axis of flow within the aorta itself approximately 1 cm above the valve plane, estimates of stroke volume, cardiac index, and regurgitant volume and fraction can be obtained. MRI can also evaluate the thoracic aorta to exclude aneurysm or coarctation at the time of evaluation, as well as provide left ventricular chamber size and functional assessment. Although cine MRI and retrospectively gated cine CT are not as readily used in clinical practice, both techniques can provide estimates of valve opening area that have been shown to correlate well with similar data derived with transthoracic and transesophageal echocardiography.
Surgical intervention is necessary in patients with symptomatic aortic stenosis, if the valve area is less than 0.6 cm 2 or if moderate aortic regurgitation develops. The choice of mechanical versus bioprosthetic valve takes into account the risk from the required lifelong anticoagulation with mechanical valves, balanced against the risk from the anticipated need for reoperation with bioprosthetic valves 10 to 15 years after implantation. Traditional nonsurgical approaches to treatment of trileaflet aortic stenosis are often inadequate for patients with bicuspid valves. Balloon valvuloplasty is generally not optimal in the setting of the heavy valvular calcification that is frequently seen in bicuspid disease, and the procedure is complicated by recurrent stenosis or the development of progressive valvular regurgitation. Percutaneous valve replacement is becoming more readily available in those patients who are not surgical candidates, but patients with a bicuspid valve are not considered optimal candidates because of the abnormal morphology of the valve orifice, as well as frequent underlying coexisting aortic root dilatation.
Surgical correction in symptomatic pediatric and young adolescent patients with a bicuspid aortic valve is often accomplished through the Ross procedure, named after the British surgeon Donald Ross. The operation consists of replacement of the aortic valve using an autologous pulmonary homograft, reimplantation of the coronary arteries into the homograft, and reconstruction of the right ventricular outflow tract with a cadaveric cryopreserved allograft. This procedure is ideal for developing children and adolescents because the allograft has been shown to grow proportional to the growth of the patient and obviates the need for anticoagulation. Investigators have also suggested that this procedure may be associated with a relatively decreased long-term incidence of neoaortic stenosis. The most common long-term complications of the Ross procedure requiring reoperation are homograft stenosis, neoaortic valve regurgitation, and aneurysm formation, as well as stenosis or regurgitation of the pulmonary allograft. Neoaortic root dilatation may be observed postoperatively; however, this condition usually stabilizes during the first postoperative year.
In cases of ascending aortic or root aneurysm and bicuspid aortic valve stenosis, concomitant aortic root replacement is performed at the time of valve replacement if the maximum diameter is 4.5 cm or greater. In the setting of bicuspid aortic valve stenosis and ascending aortic dissection, valve replacement and ascending aortic and root replacement (Bentall or modified Bentall procedure) are performed. In patients with normally functioning bicuspid aortic valves and ascending aortic dissection, valve-sparing aortic root replacement may be considered.
Atrial Septal Defects
A congenital opening in the interatrial septum is one of the most common types of congenital heart disease, occurring in approximately 1 of 1000 live births. Most patients are asymptomatic, and this explains why young adults may present with a previously undiagnosed atrial septal defect (ASD). Other patients are identified by a detectable cardiac murmur, systemic embolic events, or, in the setting of long-standing shunting, pulmonary hypertension and right-sided heart failure. ASD results from abnormalities in the complex embryogenesis of the interatrial septum, with the normal septum developing through the apposition of the septum primum, a thin membrane originating in the roof of the primordial atrium, and the septum secundum, a crescentic membrane growing from the ventrocranial wall of the primitive atrium. A large defect in the central portion of the septum secundum, the foramen ovale, is normally sealed by the thin membrane of the septum primum to create an oval indentation in the atrial septal wall referred to as the fossa ovalis. This anatomy is important to understand because the septum primum, forming the floor of the fossa ovalis, may be imperceptibly thin on CT and MRI images and can be easily misinterpreted as a septal defect when slice thickness exceeds 2 mm.
In approximately 25% of the population, the foramen ovale remains probe patent, meaning that the membranes of the fossa ovalis can be separated. In this situation, the higher left atrial pressures keep the membranes apposed, but in certain situations, such as during the Valsalva maneuver, the right atrial pressures can increase enough to open the fossa ovalis transiently. Therefore, these patients are at risk for systemic embolic events. For unclear reasons, patent foramen ovale also has an association with migraine headaches.
A probe-patent foramen ovale (a potential risk factor for stroke) or ASD may be seen as contrast material extending across the atrial septum from the right atrium to the left atrium, on power injection of intravenous contrast, or as a dephasing jet across the interatrial septum on bright-blood gradient echo MRI images ( Fig. 20-2 , A and B ).

The three main types of ASD are ostium secundum, ostium primum, and sinus venous defects. The fourth type of ASD is the unroofed coronary sinus, which is the rarest type, although it is not really a defect within the atrial septum (but has the same physiologic consequences). Failure of the septum primum to seal the foramen ovale completely results in an open communication between the atria at the level of the fossa ovalis known as an ostium secundum–type ASD (see Fig. 20-2 , C and D ). This type of ASD is the most common in the general population, and it comprises approximately 70% of lesions. Although secundum ASD is usually an isolated lesion, it can coexist with many other forms of congenital heart disease and has an association with mitral valve prolapse. Two of the more common associations are with mitral stenosis (Lutembacher syndrome) and with radial ray shortening (Holt-Oram syndrome).
Accounting for approximately 20% of ASDs, the ostium primum lesion occurs inferiorly between the fossa ovalis and the atrioventricular (AV) valve plane (see Fig. 20-2 , E ). Ostium primum ASDs are frequently encountered as part of the AV canal or endocardial cushion defects (ECDs). Seen most commonly in patients with trisomy 21 and heterotaxy syndromes, ECDs are made up of an ostium primum ASD, an inlet ventricular septal defect (VSD), and a variety of AV valve abnormalities. As a result of this frequent association with more complex congenital heart disease, ostium primum lesions tend to come to clinical attention during childhood.
The sinus venosus ASD (see Fig. 20-2 , F and G ) comprises most of the remaining ASDs. Although a few of these defects occur at the junction of the inferior vena cava (IVC) and right atrium (inferior type), most lesions are superior in location, found at the junction between the SVC and the right atrium. Superior sinus venosus ASD has a high association with partial anomalous pulmonary venous return (PAPVR), usually of the right upper lobe, to the SVC. In conjunction with this associated lesion, patients with sinus venosus ASD have a higher likelihood of developing pulmonary hypertension. A rare lesion, often referred to as an unroofed coronary sinus, may be encountered that results from a failure of separation of the superior wall of the coronary sinus with the left atrium. This lesion is challenging to identify on imaging studies and is frequently associated with a persistent left SVC. Because most of the shunting is from left to right, the coronary sinus tends to be larger than expected, with a left-sided SVC alone, a useful clue to this rare entity.
Another malformation of the interatrial septum is the atrial septal aneurysm, which results from excessive mobility of the septum with ballooning toward either the right or left atrial chamber. Atrial septal bowing is referred to as an aneurysm when it extends more than 1 cm beyond the plane of the fossa ovalis or when the overall excursion during the cardiac cycle is greater than 1.5 cm. Patients with atrial septal aneurysm have a significantly increased incidence of embolic stroke, especially if the septal aneurysm is associated with a patent foramen ovale. The reason for this is unknown and may be related to thrombus formation in the saclike segment, paradoxical embolization through the patent foramen ovale, or atrial arrhythmia. Septal aneurysm may be associated with patent foramen ovale, ASDs, and mitral valve prolapse.
Echocardiography (either transthoracic or transesophageal) is the study of choice for evaluation of suspected ASDs. Given the high spatial resolution and ability to assess blood flow with color Doppler imaging, echocardiography is ideal for the evaluation of most types of ASD. Normally filtered by the pulmonary circulation, intravenously injected agitated saline contrast can be employed with and without the Valsalva maneuver to assess for transeptal transit of saline bubbles, which are abnormally detected within the left atrium in patients with patent foramen ovale or ASD. Estimates of pulmonary arterial pressure and right-sided heart function can also be obtained with echocardiography. Because of the proximity of the transducer to the atrial septum, transesophageal echocardiography is sensitive for detection of ASD. However, detection of sinus venosus ASD can be difficult, given the superior location of most lesions. Unroofed coronary sinus ASDs are frequently occult on echocardiography.
Cardiac MRI can serve as a method of primary detection or secondary evaluation of patients with suspected or known ASD. A dephasing jet can be detected on cine steady-state bright-blood imaging sequences. In patients without coexistent pulmonary hypertension, the dephasing jet is directed toward the right atrium as a result of higher left atrial pressures. However, in patients with a patent foramen ovale or elevated right-sided heart pressures, the dephasing jet may be bidirectional or even directed toward the left atrium. Large ASDs are usually readily apparent as a result of the lack of septal tissue, but they may not display a focal dephasing jet because of the unrestricted flow. In these cases, only mild low-velocity turbulence may be seen. An estimate of the diameter of the defect should be reported, as well as the presence or absence and minimal width of the circumferential rim of atrial septum, because this information is useful in evaluating transcatheter occluder device appropriateness and size.
In all patients with suspected ASD, flow quantification of the aorta and pulmonary arteries is required to assess the degree of shunting by comparing the forward flow across the pulmonic valve with the forward flow across the aortic valve (Qp/Qs). Care should be taken when setting the cross-sectional flow quantification sequences that the plane of imaging is truly orthogonal to the long axis of flow in each vessel, because off-axis imaging in one or both vessels can lead to false shunt estimates. Unlike echocardiography, during which assessment of right ventricular function can be restricted by retrosternal location and limited acoustic window, MRI can qualitatively and quantitatively assess right heart chamber size and function. Intravenous contrast material is not required for evaluation. If pulmonary hypertension is present, pulmonary arterial enlargement, right ventricular and atrial dilatation, right ventricular hypertrophy, tricuspid regurgitation, and interventricular septal dyskinesis may be seen. Care should be taken to identify all pulmonary veins and their course to the left atrium, best accomplished on multiplanar noncine SSFP bright-blood sequences, to exclude partial anomalous venous return, which is frequently seen in patients with superior sinus venous defects. Given that right upper lobe partial anomalous venous return can be challenging to identify, intravenous contrast may be used to assist in the detection of all right upper lobe veins.
Although CT is not a primary method of evaluation of ASD, these lesions can be seen on contrast-enhanced imaging studies performed for noncardiac reasons or during the evaluation of other cardiac diseases. Detection depends on the lack of septal tissue, which can be seen in large defects, or the presence of transeptal contrast flow, which requires differential degrees of opacification of the right and left atrium. Patent foramen ovale can be seen when high-density contrast material crosses the fossa ovale, frequently directed at a slight angle or parallel to the interatrial septum as it courses between the separated membranes at the fossa ovalis. Morphologic changes in keeping with pulmonary hypertension, including pulmonary arterial enlargement and right heart chamber size and ventricular hypertrophy, can be seen. Multidetector CT (MDCT) is also highly sensitive for detection of anomalous pulmonary venous drainage because of its superior isovolumetric spatial resolution.
In the adult patient, ASD and symptomatic patent foramen ovale are most commonly treated with percutaneous septal occluder devices, and open surgical procedures are reserved for patients with very large defects, unsuitable margins of a defect, or associated lesions requiring corrective intervention. In young children, surgical patch repair or primary closure is typically required. Sinus venosus defects are not amenable to occluder device placement and require surgical correction, which often includes baffling of associated anomalous pulmonary venous drainage back to the left atrium.
Atrioventricular Septal Defects
Fusion of the dorsal and ventral endocardial cushions gives rise to the ostium primum portion of the interatrial septum, the inlet portion of the interventricular septum, and the septal portions of the AV valve leaflets during the sixth week of embryologic development. Abnormal fusion or lack of fusion of the endocardial cushions results in a range of abnormalities known as ECDs, which include ostium primum ASD, inlet VSD, and AV valve abnormalities. A wide range of valvular abnormalities can be seen from a common AV valve to deformities of tricuspid or mitral septal leaflets. The relative position of the defects and the type of valve abnormality leads to various presentations, depending on the resulting direction of systemic and pulmonic blood flow.
Echocardiography readily identifies this defect in infants and even prenatally. In addition to shunting identified on color Doppler flow and an absence of portions of the interatrial and interventricular septa, the tricuspid and mitral valve planes are abnormally located along the same axis in ECDs. Normally, the tricuspid valve is slightly more apical in location with respect to the mitral valve. The types of valvular abnormalities can also be characterized.
MRI is often used in older patients with ECDs (adolescents and young adults) before and after surgical intervention (see Fig. 20-2 , E ). In addition to identifying the abnormalities described on echocardiography, MRI allows for flow quantification of the aorta and pulmonary artery to assess the direction and degree of shunting (Qp/Qs). Quantitative biventricular functional analysis is also performed, and the anatomic relationships of the defects to other cardiac structures such as the left ventricular outflow tract are noted. MDCT can play a complementary role, with its strength lying in multiplanar demonstration of the anatomic defects and relationships at the cost of radiation exposure.
Because shunting is often predominately from left to right, increased pulmonary arterial flow, if left untreated, eventually may lead to Eisenmenger syndrome. Surgical correction is usually performed at 4 to 6 months of age or earlier if the patient is symptomatic in spite of medical therapy. Temporizing measures before definitive surgical correction are banding of the pulmonary artery to decrease pulmonary flow and ASD closure using a pericardial patch. If a complete AV canal exists, the double patch technique is often used. In this operation, the atrial defect is again closed with a pericardial patch, the VSD is closed with a synthetic patch, and the malformed AV valve leaflets are reconstructed and sandwiched between the atrial and ventricular septal patches.
Given that the AV node and bundles of His are in close proximity to the surgical field, conduction anomalies including AV block are common complications of repair. This can complicate ECG-gated MRI cine bright-blood sequences or even preclude MRI altogether if a non–MRI compatible implantable pacer device is required. On follow-up imaging, attention to residual ASDs or VSDs is necessary, including flow quantification of the aorta and pulmonary artery. Qualitative tricuspid and mitral valve evaluation for regurgitation should be performed. Aortic outflow tract obstruction can also occur.
Ventricular Septal Defects
After the bicuspid aortic valve, VSD is one of the most common forms of congenital heart disease. VSDs can be isolated as a result of deficiency in septal tissue, part of a more complex congenital heart disease, or even acquired through penetrating injuries or myocardial infarction. VSDs are categorized according to their physiologic consequence and anatomic location. Physiologically, VSDs are classified as restrictive if they are flow limited and result in a significant pressure gradient between the left and right ventricles. Because these lesions are small and restrictive to flow, they do not result in significant shunting, but they do predispose patients to endocarditis, for which antibiotic prophylaxis before invasive procedures is required. Nonrestrictive VSDs are larger lesions that permit hemodynamically significant shunting and as a result are symptomatic, initially from right ventricular overload and over time from the development of irreversible pulmonary hypertension (Eisenmenger syndrome).
VSDs are also classified according to anatomic location. Accounting for the overwhelming majority of defects in adults, perimembranous VSDs occur in and extend from the membranous portion of the interventricular septum, which lies immediately beneath the aortic valve and is in continuity with the base of the septal leaflet of the tricuspid valve ( Fig. 20-3 , A to C ). The most common defect in children and the second most common defect in the adult population, muscular VSDs are bordered entirely by myocardium and occur along the muscular interventricular septum (see Fig. 20-3 , D and E ). Many muscular VSDs spontaneously close in early childhood, a characteristic accounting for the finding that VSDs are more common than ASDs in this age group, yet ASDs are more commonly encountered in young adults. Most acquired VSDs are muscular.


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree