(1)
Neuroradiology, Universityhospital Würzburg, Würzburg, Germany
4.1 Imaging Techniques
MRI is clearly the method of choice for the imaging of diseases of the CNS. CT usually has only an additional role in case of emergency or contraindications against MRI. For radiotherapy planning the physical properties of CT are essential [114]. However, it still is the only method for a reliable depiction of calcifications near the skull base, which can have a diagnostic potential in some tumor entities [115, 116]. The signal intensities on MRI and density values on CT allow a limited diagnosis of the histology of the tumor (see Chap. 3). Invasive angiography or conventional radiography is useful only in rare exceptions.
4.1.1 Standard MRI Technique (Proposal for the SIOP-E Tumor Trials)
Additional to the standard SE sequences, an increasing number of various sequences have become available. However, the imaging characteristics on these sequences and thus the size of tumors might vary artificially. Key to a correct evaluation of study patients in a comparable way is to keep standardized imaging sequences during follow-up. A standard imaging protocol should contain T2/FLAIR (or proton density PD) sequences. FLAIR alone is not sufficient, because the cell density of a tumor cannot be sufficiently evaluated without a T2-sequence. The only performance of a T2 or FLAIR bears the risk to miss pathology (Fig. 4.1a–c). They should be combined with T1-weighted sequences before and after intravenous (i.v.) administration of contrast material. For children, the slice length should not exceed 4 mm. For small structures even much smaller slice lengths might be necessary. Although many surgical or radiotherapy treatment planning systems require three-dimensional sequences, these should only be additional to the core of standard imaging, because the contrast behavior of tumors on SE series and on 3-D-MPR series can differ considerably [117] making a comparison impossible (Fig. 4.1f–i). An automatized 3-D volume calculation of brain tumors can only be used in single or limited center studies because the acquisition parameters have to be uniform.








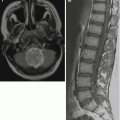
Fig. 4.1
(a–i) T2 and FLAIR of the brain are necessary for complete evaluation. While on FLAIR (a) there is no suspicion of dissemination, leptomeningeal deposits in the cerebellar sulci are clearly seen on T2 (b). On an enhanced T1 (c), the leptomeningeal dissemination is not detectable as well. Note the nonenhancing primary tumor (MB) in the fourth ventricle. But it may also be vice versa and it cannot be predicted in the individual case. In a child with an ependymoma the histologically verified metastases in the cerebral sulci are only visible FLAIR (d) but not on T2 (e). The comparison of a precontrast SE image (f) with a postcontrast MPR (g) for the definition of an enhancing residual tumor may be hard or even impossible. The tiny spinal metastasis is not visible on the 1 mm thick MPR sequences (h) while it can be clearly seen on the thicker turbo SE (TSE) series (i) done during the same examination
Diffusion weighted MRI (DWI) with the additional calculation of the ADC allows not only the depiction of infarcted brain but also an estimation of cellular density in the absence of hemorrhage [118]. Together with the signal intensity on T2-weighted MRI the ADC is a very useful tool for differential diagnosis [119]. Susceptibility weighted sequences (SWI) are useful for the identification of calcifications or blood degradation products. However, in pediatric brain tumors with the exception of craniopharyngiomas this feature is of little importance (see also Fig. 3.9f) compared to its value in the differentiation of adult high-grade gliomas [115, 116]. As SWI sequences are prone to disturbances by susceptibility artifacts the skull base is not a useful region.
4.1.2 Early Postoperative Imaging
A residual tumor after resection can only be identified within the first 3 days after resection because after this time period a nonspecific reaction of the brain to the surgical trauma can create contrast enhancement which is virtually indistinguishable from residual tumor [120] (Fig. 4.2a–c). Unfortunately also during and very early after resection surgery induced enhancement may cause problems in the identification of a possible residual tumor especially in case of the use of electrocoagulation [121] (Fig. 4.2d–g). Therefore and because of frequent increased artifacts induced by air in the intracranial cavity (Fig. 4.2h, i), we do not advice to enter the MRI directly from the operating theatre to perform the early postoperative MRI, which seems very attractive in terms of logistics. Ideal for the early postoperative MRI are day 1 and 2 after surgery. However, if in case of a contrast enhancing tumor this time period is missed, then the correct identification of a residual tumor might not be possible for a long time or even forever (Fig. 4.2a–c). Nonenhancing tumors can only be identified on the basis of their T2/FLAIR or PD characteristics. Therefore, the comparability of MRI to the preoperative time point is of utmost importance. Also a change of magnetic field strength is problematic and should be avoided for the pre- and postoperative comparison and also for further follow-up.






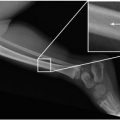
Fig. 4.2




(a–i) If the early postoperative time period is missed, postsurgical effects might mimic an enhancing residual tumor (a, morphology of a medulloblastoma on preoperative enhanced T1-weighted MRI; b, regular, early-postoperative MRI on day 1, enhanced T1-weighted image without residual tumor; c, enhanced T1-weighted MRI on day 8 after surgery with nonspecific postoperative enhancement). On very early postoperative MRI immediately after surgery, false positive enhancement possibly after coagulation during surgery may mimic a residual tumor (d, preoperative postcontrast T1-weighted MRI of a pilocytic astrocytoma in the vermis cerebelli; e, f T1-weighted pre- and postcontrast MRI immediately after surgery showing a lot of marginal enhancement also in parts of the tumor without preoperative enhancement, which is completely resolved without further treatment on the first control after 3 months (g)). Intracranial air can lead to heavy susceptibility artifacts on very early postoperative MRI. The sagittal T2-weighted SE image (h) shows a complete air filling of the ventricles and the superior parts of the subarachnoid spaces. This leads to a massive effacement on T2* images (i)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree