13 Imaging in Chronic Spinal Cord Injury: Indications and Benefits
Abbreviations
AXR abdominal radiograph
US ultrasound
IVP/U intravenous pyelogram/urogram
MRI magnetic resonance imaging
CTM computed tomography myelogram
ERPF effective renal plasma flow study (Tc-99m MAG3)
DREZ dorsal root entry zone
PPMM progressive posttraumatic myelomalacic myelopathy
PPCM progressive posttraumatic cystic myelopathy
PCNL percutaneous nephrolithotomy
EO ectopic ossification
HO heterotopic ossification
PAO paraarticular ossification (osteopathy)
DVT deep venous thrombosis
SCI spinal cord injury
Introduction
With improvements in the management of patients with spinal cord injuries (SCI) over the past 60 years, the demographics have changed quite markedly.1 With patients injured in their youth now surviving for many decades, there is an aging population of patients with SCI so the chronic changes in the body, particularly in the central nervous and renal systems, are becoming more important. In addition, there is a group of geriatric patients injured as a result of trauma exacerbating degenerative changes in the spine.
As the late complications of SCI are seen increasingly frequently, regular surveillance of both the renal tract and the central nervous system is important as the treatment of impending, potentially fatal complications can be implemented before damage has progressed too far.
| Routine surveillance | Central nervous system Renal tract |
| Specific conditions | Deteriorating neurology Spinal instability The acutely unwell patient Deteriorating renal function Pressure sores Ectopic ossification Pain Muscular spasm Airway problems Elective management of the renal tract |
Renal tract complications are particularly dangerous as they are often clinically silent but regular surveillance to detect early deterioration in renal function, particularly from reversible causes such as reflux or obstruction, can preempt problems. Follow-up protocols depend on the bladder management regimen but most centers advocate regular ultrasound with less frequent isotope function studies and urodynamics.
With the increasing ability to diagnose and treat the neurological complications, surveillance of the state of the spinal cord with MRI is also important and many centers now advocate checks every few years with sagittal mid-line T2W sections generally sufficient.
Imaging is critical in acute situations. In addition to suffering from the usual conditions, patients with spinal cord injury suffer others peculiar to, or particularly related to, the injury, which may be missed as their symptomatology is greatly altered by their paraplegic or quadriplegic status and they may often present as generally unwell but with no obvious cause or pathological focus.
This chapter discusses the role of radiology in routine surveillance of the CNS and the renal tract as well as in assessing specific conditions such as deteriorating neurology or renal function, pain, spinal instability, pressure sores, ectopic ossification, muscular spasm, spinal instability, airway problems, and elective operations on the renal tract (Table 13.1).
Neurological System
Significant recovery of neurological function rarely commences more than 1 year after the accident, particularly in complete lesions. There is often, however, deterioration with time, the main heralding symptoms being loss of function, the loss of a reflex, or increasing pain or spasms. Any deterioration, however slight, in the limited function the patient possesses may make the difference between relative independence and the need for chronic care, so prompt and accurate diagnosis as well as regular surveillance to preempt problems is of paramount importance.
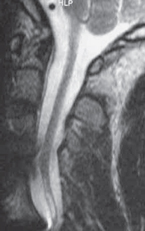
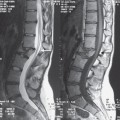
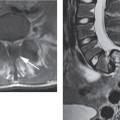
Fig. 13.1 Sagittal T2W section showing atrophy.
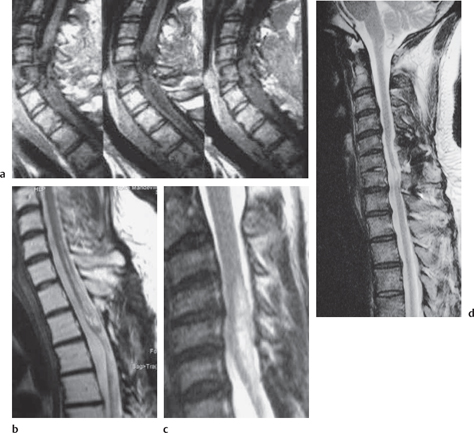
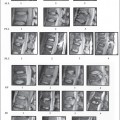
Fig. 13.2 Progressive posttraumatic myelomalacic myelopathy. a Sagittal T1W sections showing changes at 3 months post-injury. b Sagittal T2W section showing a mixed signal change in the cord. c Sagittal T2W section showing posterior arachnoid tethering. d Sagittal T2W section showing myelomalacia.
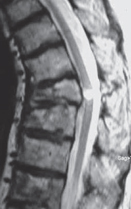
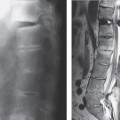
Fig. 13.3 Sagittal T2W section showing a focal cyst at the injury site.
Cord Changes Post-SCI
Although in 1915 Holmes described six SCI patients whose neurological condition had deteriorated and who at surgery had intramedullary cysts extending several segments above or below the injury site, until MRI became available, there were few further reports of the changes.2
The three most important changes that have been noted in the cord are atrophy (Fig. 13.1), myelomalacia (Fig. 13.2; progressive posttraumatic myelomalacic or noncystic myelopathy [PTMM]), and cystic changes (progressive posttraumatic cystic myelopathy [PTCM], focal cyst [Fig. 13.3], and syringomyelia [Fig. 13.4]).
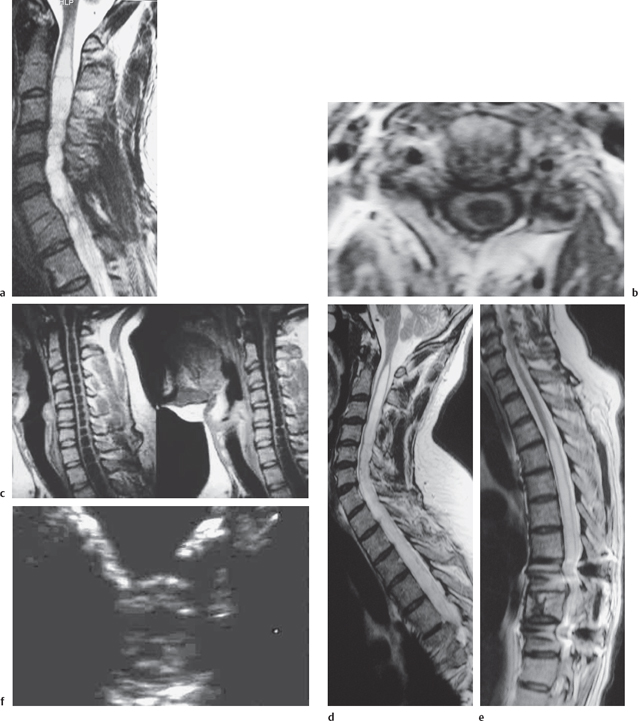
Fig. 13.4 Syringomyelia. a Sagittal T2W section showing a flare at the rostral end indicating edema. b Axial T1W section. c Sagittal T1W sections showing “septa” that are probably decussating tracts. d Sagittal T2W section of a syrinx. e Posterior fixation clearly showing a syrinx. f Intraoperative US axial image showing the cyst.
Understanding of all these conditions has been greatly impeded by a general lack of agreement on their definitions, both histologically and radiologically, in the various studies of their occurrence, and a true incidence is essentially unknown. Posttraumatic changes in the cord are difficult to study as there is no good animal model and there are few extensive patient series. The most comprehensive descriptions and insights into the pathophysiology are by Quencer and Squier.3–5
Wallerian degeneration with demyelination and subsequent gliosis is the main cause of irreversible proximal atrophy causing a loss of spinal cord substance and abnormal narrowing of the cord diameters. Other factors are still poorly understood and possibly relate to ischemia and focal inflammatory changes, a postulated mechanism that surgeons who decompress or physicians who administer high-dose corticosteroids within 6 hours of trauma are aiming to ameliorate.6–8
At the time of injury there may be an injection of crushed cord tissue from the site of maximum impact along shear planes in the cord. The cord then becomes edematous and the tissue undergoes necrosis and cyst formation, a process also probably involving liquefaction of parenchymal hematoma, ischemia, the release of intracellular enzymes, and mechanical damage from cord compression. Those cysts that develop macroscopically are eccentrically placed, are rarely contiguous with the central canal, and have been termed “contusion evolving to cavity.”
The progression to syringomyelia, i. e., a cyst extending beyond the limits of bony damage, appears to involve arachnoid adhesions, presumably secondary to blood products and inflammatory changes at the time of injury. The inability of the cord to move freely and the consequent turbulent CSF flow dynamics, with high-pressure ballistic impulses particularly focused on the damaged parts of the cord, is thought to lead to the formation and propagation of the syrinx,9–11 though in-vitro hydrodynamic studies are still incomplete and inconclusive. This leads to a “pre-syringomyelic” myelopathic state of reversible cord edema that develops into the two distinct conditions of PPMM and PPCM.12–14 The radiological appearances may oscillate rapidly over a few weeks with no real contemporary correlation with the clinical picture (as shown by Milhorat in 1998 and from personal unpublished experience).15 The histological changes are unknown.
CSF flow assessments in patients with Chiari malformations and post-traumatic cysts support these views and restoration of CSF dynamics with untethering of adhesions and an allograft expansile duraplasty is the basis of the most successful surgical approach for treatment,16–23 shunting alone being less successful (Fig. 13.5).
The need for early operative restoration of normal alignment and the reduction of deformity, and restoration of canal capacity to normalize CSF function is still controversial with most studies being retrospective on symptomatic patients but there is a large body of conservative thinkers who feel that the risks of surgery for most patients do not often outweigh the risks of subsequent neurological change.24–26
Reported Series
Atrophy
Cord measurements at different levels have been determined from pathological studies and CT myelography although the results are significantly different between the two methods depending on the window/ level settings. Similar differences exist in measuring atrophy on T1 and T2 sequences (personal observations). From the pathological studies, a normal cervical cord A-P diameter is not less than 7.5 mm, and the thoracic cord 6.5 mm.36 Some authors have assessed atrophy qualitatively so it is difficult to compare rates when the criteria are not given.
Using MRI, incidences of 7%,37 18 %,25 and 27% have been reported but the groups are heterogeneous and symptomatic and the techniques undefined so comparisons are virtually meaningless.36
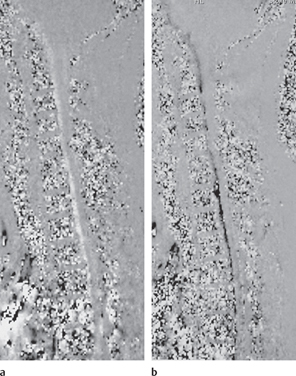
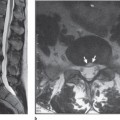
Fig. 13.5 CSF flow study. The white (a) and black line (b) anterior to the cord is the pulsating CSF. There is no significant flow posteriorly indicating that the CSF is not moving, presumably as the result of posterior adhesions.
Myelomalacic changes
In the literature there is often confusion between cysts, myelomalacia, and syringomyelia so comparisons of the different series is impossible. Some authors record all abnormal cystic-looking structures as posttraumatic spinal cord cysts (PTSCC),38,39 however others distinguish between focal cysts, syrinxes, and myelomalacia.25,36,37,40 Incidences for “syringomyelia” vary between 1%, 1.4%, and 3.2% using clinical criteria in the pre-MRI era and up to 59% using MRI.39 The term “myelomalacia” has been used loosely to describe acute ischemic changes due to vascular stasis and perivascular edema or areas of degenerative change in the cord in which there are multiple small cysts and gliosis.
Examining a heterogeneous group of 87 patients, approximately one-third of whom had already undergone spinal surgery, Curati et al defined myelomalacia as areas of patchy low intensity on T1W images with corresponding high intensity on T2W images with signal intensities not quite the same as the surrounding CSF and syrinx as a low intensity linear or septate structure within the cord tissue on T1W sections together with areas of low signal in at least part of the cyst on the T2W sections—a change thought to be due to fluid motion—the overall signal characteristics being similar to those of the surrounding CSF.25 No distinction between syringomyelia and a focal cyst was made. With these criteria, they found an incidence of myelomalacia in 37%, syringomyelia in 40%, atrophy in 18%, and persistent cord compression in 32%. This latter was found in approximately equal frequency in patients with total paraplegia, incomplete paralysis, and with minor signs only.
The confusion and heterogenicity in published series emphasizes the need for long-term, longitudinal, prospective studies to try to understand the pathogenesis of these entities.41
To date the most likely indicator of incidence is the study reported by Wang et al,42 in which MR imaging was performed on a cohort of 153 consecutive patients, regardless of their symptomatology, who had had their SCI at least 20 years previously. Using the following definitions:
Atrophy: abnormal narrowing of the spinal cord in the sagittal plane two segments or more beyond the limits of the vertebral injury (<7.5mm in the cervical and < 6.5 mm in the thoracic cord—based on normals taken from measurements on a local population; Fig. 13.1).
Myelomalacia: an area of low T1W and high T2W signal intensity that is between that of normal spinal cord and the surrounding CSF with ill-defined contours and irregular shapes, occasionally appearing as a string of small cysts (Fig. 13.2).
Syrinx: an area with the same signal intensity as CSF which is usually tapered at one or both ends and which may appear loculated, with a well-defined contour and which extends beyond the length of maximal bony damage (Fig. 13.4).
Focal cyst: an area having the same signal intensity and contour definition as a syrinx, but with a round or oval shape and confined to the site of maximal bony protrusion into the spinal canal (Fig. 13.3).
Disruption: the complete absence of the spinal cord signal through the traumatized area.
They reported an incidence of
– atrophy in 62%,
– myelomalacia in 54%,
– syringomyelia in 22%,
– focal cysts in 9%,
– and disruption in 7%.
In a stable cyst or syrinx there is fluid equilibrium and in our experience, appearances may not change for many years though if there is no deteriorating neurology, few surgeons would recommend an operation. Life-style modification may be suggested, e.g., the avoidance of contact sports, which might aggravate the condition by increasing the ballistic impulses of the CSF.
In a hydrodynamically unstable cyst, however, there may be MRI abnormalities in the cord tissue surrounding the cyst. An increased T2W signal cephalad to the top of a syrinx (Fig. 13.4a) has been correlated with clinically progressive disease and its disappearance with resolution of the clinical problems post-operatively. This presumably represents edema from raised intra-cystic pressure and is analogous to the flare in the periventricular edema of hydrocephalus.43,44
Treatment
The treatment of syringomyelia is still controversial and generally reserved for symptomatic patients.27,28 CT-guided cyst puncture as a temporary and predictive treatment has been described but most surgery to date has been myelotomy or shunt placement, sometimes with the aid of intraoperative US for restoration of CSF flow dynamics, which is now the favored approach.
Omental transposition and grafting has largely been discredited in controlled trials but a Chinese experience of 3000 patients claims return of some useful autonomic function.29–31
In refractory cases, cordotomy is offered but there is a general reluctance to accept this final step in the light of impending cord treatments aimed at reconnecting the pathways and promoting regeneration of the cord (see Chapter 17).
Post-operative assessments to confirm syrinx collapse or a return to normal of the cord signal can be rapidly performed in a brief examination as only two or three T1W or T2W sagittal mid-line sections are required. Occasionally shunt blockage is suspected and i. v. contrast enhancement may show chronic inflammatory reaction and perhaps infection around the tip (Fig. 13.6).
Imaging
The purpose of imaging in the chronic state is to assess the presence of remediable pathology. PPMM, PTCM, and syringomyelia are the only directly treatable complications but other pathologies may coexist, such as disk disease, tumors etc., and they would require as full a work-up as in any other patient.
Frequency of Investigation
Sequential studies have shown that changes in the cord can progress at various times and with surprising speed. A developing syrinx may become evident within 2-3 months (Fig. 13.2a
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree