and Daniel Thomas Ginat3
(1)
Department of Radiology, Beverly Tower Wilshire, Advanced Imaging, Beverly Hills, CA, USA
(2)
University of Southern California, Keck School of Medicine, Los Angeles, CA, USA
(3)
Department of Radiology, University of Chicago, Pritzker School of Medicine, Chicago, IL, USA
1.1 Overview of Facial Cosmetic Materials and Their Imaging Features
A wide variety of materials have been used to augment facial tissues in the form of implants, grafts, fillers, and injectables (Fig. 1.1). The main types of implant and graft materials (Table 1.1) include solid silicone, polytetrafluoroethylene, high-density porous polyethylene, bone, and fat, while the main types of fillers and injectables (Table 1.2) include hyaluronic acid preparations, calcium hydroxyapatite, collagen, polytetrafluoroethylene, silicone, alkyl-imide gel polymer, and botulinum toxin, among others.
On occasion, CT or MRI will be obtained to evaluate complications, which include foreign body granuloma formation, seroma, infection/fistula/draining sinus, skin atrophy, implant migration and extrusion, change in cosmetic result, functional alteration, vision loss, dysesthesia, ossification, and obstructed breathing, among others, depending on the type of implant or graft. Alternatively, changes related to facial surgery may be encountered incidentally on imaging.
1.2 Forehead Augmentation
1.2.1 Discussion
Forehead augmentation is performed for improving the upper facial contour. A variety of alloplastic implants have been used for this purpose, including polytetrafluoroethylene and silicone. Often, silicone implants have corrugated edges and central perforations in order to optimize fixation and prevent capsular contraction. Fillers, such as calcium hydroxyapatite, also have a role in forehead augmentation. These materials can be inserted in the midline (Figs. 1.2 and 1.3), lateral brow (Fig. 1.4), or both. Botox is another minimally invasive option for reducing lines and wrinkles.

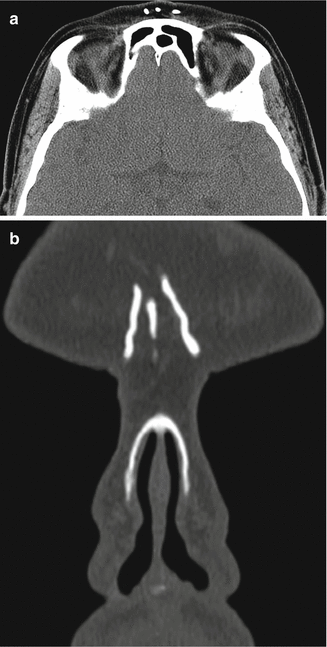
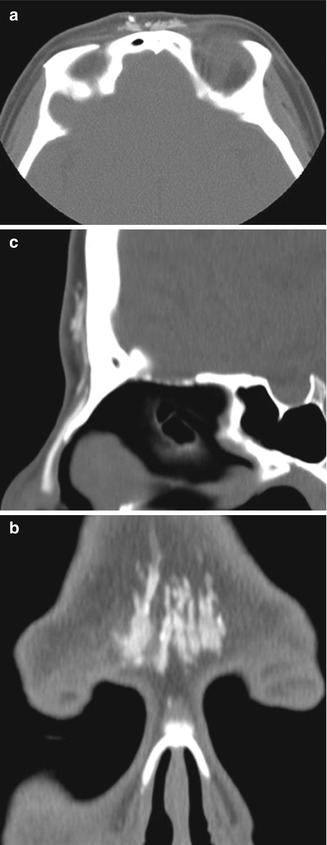
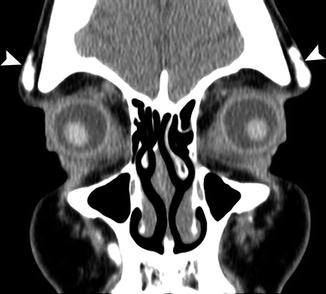

Fig. 1.1
Photographs of various facial implants (a, b)
Table 1.1
Implants and grafts
Material | Properties and uses | Imaging appearance |
|---|---|---|
Solid silicone | Rubber elastomer used since 1956 | CT: variable attenuation, usually more hyperattenuating than soft tissue, but less hyperattenuating than bone and best discerned using bone windows |
Well-tolerated | MRI: very low signal intensity on T1- and T2-weighted sequences | |
Indications: chin, lateral jaw, cheek, and nose augmentation | ||
Polytetrafluoroethylene | Long-lasting, but can be removed surgically | CT: higher attenuation relative to soft tissues, but lower attenuation than bone |
Indications: lower face-lift, nasal, and forehead augmentation | MRI: hypointense to fat on T1- and T2-weighted sequences | |
High-density porous polyethylene | Inert and biocompatible | CT: attenuation between fat and water |
Low complication rate | MRI: hypointense to fat on T1- and T2-weighted sequences | |
Permanent | Enhancement may occur due to fibrovascular ingrowth | |
Indications: lower face and nasal augmentation. Also used for orbital and auricular reconstruction | ||
Bone | Used more frequently in the past for chin and cheek augmentation, often in the form of “button” implants | CT: same as normal bone elsewhere; cortex and trabecular can be identified unless resorption has occurred |
Bone or osteochondral grafts are sometimes used in rhinoplasty | MRI: same as bone elsewhere | |
Harvest sites include the calvarium and rib |
Table 1.2
Fillers and injectables
Filler material | Properties and uses | Imaging appearance |
|---|---|---|
Liquid silicone | Analogous to intraocular silicone injection, but not currently FDA approved for facial cosmesis | CT: variable attenuation, usually similar to soft tissue density |
Permanent agent | MRI: variable signal on T1 and T2 depending on viscosity | |
Relatively higher risk of granuloma formation, particularly with non-medical grade formulations | Decrease in signal with fat suppression | |
More conspicuous on STIR | ||
Collagen | Naturally occurring protein derived from purified bovine collagen given via a subdermal injection | CT: soft tissue attenuation; subcutaneous fat appears infiltrated |
Indications: wrinkles, scars, and lines Lasts approximately 3–6 months | MRI: same signal intensity as water (hypointense to fat on T1 and hyperintense to fat on T2); occasional minimal peripheral enhancement that can persist up to 2 months | |
Hyaluronic acid preparations | Injectable gel | CT: water attenuation; subcutaneous fat appears infiltrated |
FDA approved Indications: wrinkles, scars, and lines Lasts about 6 months and can be removed using hyaluronidase injection | MRI: same signal intensity as water (hypointense to fat on T1 and hyperintense to fat on T2); occasional minimal peripheral enhancement that can persist up to 2 months | |
Polytetrafluoroethylene | Implanted – not injected | CT: higher attenuation relative to soft tissues |
Permanent, threadlike material (not metabolized, but can be removed surgically) | MRI: hypointense to fat on T1- and T2-weighted sequences | |
Indications: filler in multiple sites (nasolabial folds, lips, glabella) | ||
Calcium hydroxyapatite | US FDA approved | CT: high attenuation (generally 280–700 HU) initially; eventually the calcium resorbs, typically incites fibrous tissue formation that may be visible on imaging |
Temporary injectable that lasts up to at least 2 years | MRI: similar to bone (hypointense to muscle on T1- and T2-weighted sequences); no enhancement | |
Indications: wrinkles, lines, scars, and HIV lipoatrophy | PET: can lead to hypermetabolic response | |
Alkyl-imide gel polymer | Injectable, biocompatible, nontoxic, nonallergenic soft tissue filler | CT: water attenuation masses surrounded by thin collagen capsule |
Uses: HIV lipoatrophy and rejuvenation | MRI: same signal intensity as water (hypointense to fat on T1 and hyperintense to fat on T2) | |
Botulinum toxin | Neurotoxin for the temporary improvement of glabellar lines | CT: nil |
Intramuscular injection (corrugator and procerus muscles; 5 sites – 0.1 ml each) | MRI: nil | |
Maximum effect at 30 days. Lasts up to 6 months |

Fig. 1.2
Mid-forehead augmentation with polytetrafluoroethylene. Axial (a) and coronal (b) CT images demonstrate hyperattenuating linear implants in the glabella

Fig. 1.3
Mid-forehead augmentation with calcium hydroxyapatite. Axial (a), coronal (b), and sagittal (c) CT images demonstrate hyperattenuating linear implants with fuzzy edges, which provide a gentle convex contour to the glabella despite the flat frontal bone. A silicone dorsal nasal implant is also present

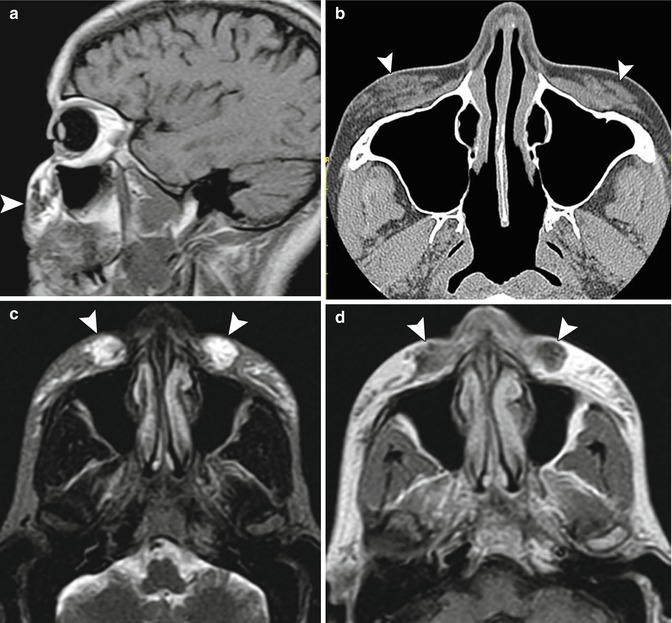
Fig. 1.4
Lateral brow augmentation. Coronal CT image shows collections of calcium hydroxyapatite in the lateral supraorbital areas (arrowheads)
1.3 Cheek and Nasolabial Fold Augmentation
1.3.1 Discussion
Cheek augmentation consists of expanding the malar region, submalar region, or a combination of these, often bilaterally. The procedure is performed for soft tissue enhancement or simply for correcting a deficient or atrophic face, including HIV lipoatrophy. A wide variety of materials have been used for these purposes, including coral implants (Fig. 1.5), silicone rubber implants (Fig. 1.6), injectable silicone (Fig. 1.7), injectable calcium hydroxyapatite (Fig. 1.8), polytetrafluoroethylene strips (Fig. 1.9), hyaluronic acid (Fig. 1.10), collagen (Fig. 1.11), alkyl-imide gel polymer (Fig. 1.12), and combination of materials (Fig. 1.13).
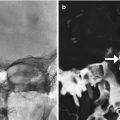
Seromas can be present and appear as simple fluid collections surrounding the implants (Fig. 1.14). Seromas typically resolve spontaneously, unless there is superimposed infection. In such cases, the patient may present with fever and purulent drainage. On imaging, stranding of the subcutaneous fat overlying the implant is often evident (Fig. 1.15). Additional manifestations of implant-associated infections include osteomyelitis and draining sinuses (Fig. 1.16). Other complications depend on the type of material used. In particular, liquid silicone can induce extensive inflammation, which appears as stranding or high T2 signal in the subcutaneous tissues (Fig. 1.17). Furthermore, injected nonmedical-grade silicone has a particular propensity to cause scars and granulomas. These complications can develop many years after injection of the filler. Hypertrophic scars can appear as bands of soft tissue within the subcutaneous fat on CT (Fig. 1.18). Granulomas often appear as subcentimeter rounded or oval foci of variable attenuation on CT (Fig. 1.19). Silicone foreign body granulomas can contain microcalcifications or form eggshell calcifications. Implants, such as silicone rubber, can occasionally erode through the bone (Fig. 1.20) and potentially result in sinusitis. Cheek implantation can sometimes induce heterotopic bone formation (Fig. 1.21). Bone grafts can resorb over time, thereby also diminishing cosmetic effect. Migration of fillers or implants can mimic mass lesions and impair vision (Fig. 1.22).
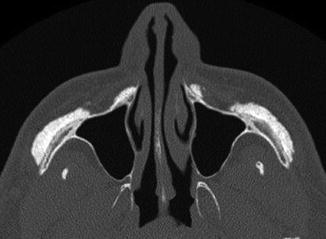
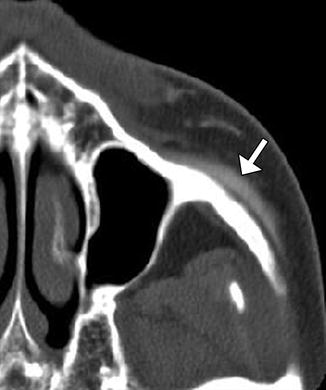

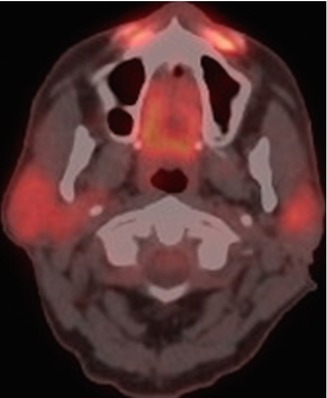
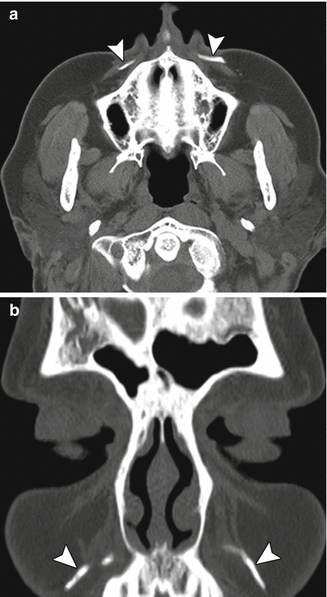
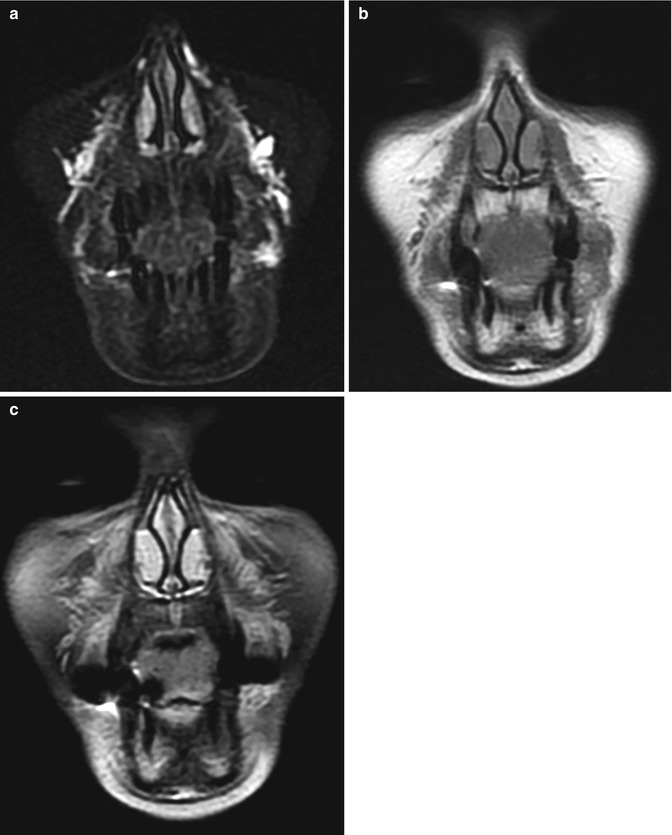

Fig. 1.5
Cheek augmentation with coral implants. Axial CT image shows hyperattenuating material overlying the bilateral malar eminences

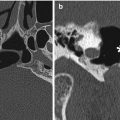
Fig. 1.6
Silicone implant cheek augmentation. Axial CT image shows bilateral crescent-shaped hyperattenuating implants (arrow) over the zygomatic and maxillary bones

Fig. 1.7
Acne scar treatment with silicone oil filler. Axial CT image shows punctate hyperattenuating foci of the filler material (arrow) within the subcutaneous tissues of the left cheek

Fig. 1.8
Anterior face and nasolabial fold calcium hydroxyapatite injection. There is hypermetabolism at the site of the nasolabial fold fillers (arrows) on 18FDG-PET/CT

Fig. 1.9
Nasolabial fold polytetrafluoroethylene filler. Axial (a) and coronal (b) CT image shows thin strips of hyperattenuating material in the bilateral nasolabial folds and subcutaneous tissues (arrowheads)

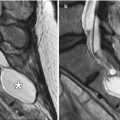
Fig. 1.10
Nasolabial fold hyaluronic acid augmentation. Coronal STIR (a), T1-weighted (b), and post-contrast fat-suppressed T1-weighted (c) MR images demonstrate streaky material with high T2 signal, as well as mild enhancement