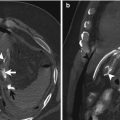
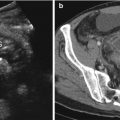
Fig. 15.1
Axial post-contrast medium CT scan shows the ascending colon thickening (white arrows) and surrounded by a pericolic fat inflammation associated with dense fluid and air bubbles (white arrow heads). Fluid film and air bubbles spread also into the abdominal wall (black arrow head)
15.5 Stomach Cancer
The most frequent CT finding of stomach cancer is represented by a focal or diffuse gastric wall thickening, with a dishomogeneous post-contrast medium wall attenuation, particularly of the adenocarcinoma type (Fig. 15.2). Other CT signs are outer and inner lobulated irregular margins, ulceration, and narrowing of the lumen with obstruction and secondary proximal gastrectasia. The areas of dishomogeneous post-contrast enhancement are due to tumoral tissue inhomogeneity for edema, necrosis, or hemorrhage [6].
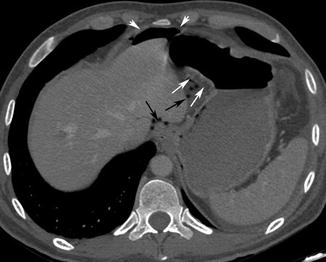

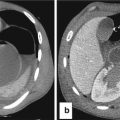
Fig. 15.2
Axial post-contrast medium CT scan shows pneumoperitoneum (white arrow heads) and a focal gastric wall thickening, with a dishomogeneous post-contrast medium wall attenuation (white arrows), representing a stomach cancer. The perforation of the lesion has been demonstrated by adjacent multiple air bubble spread within the small omentum (black arrows)
The tumor could invade surrounding organs and structures as the diaphragmatic muscle, pancreas, spleen, left lobe of the liver, transverse mesocolon, and small and greater omentum.
When the greater omentum is diffusely infiltrated, an “omental cake” may be recognized at CT scan as an extensive dishomogeneous thickened soft tissue mass, separating the colon and the small bowel from the anterior abdominal wall.
Metastasis could be evidenced at the liver, adrenal glands, lymph nodes, ovaries, and peritoneal cavity.
Gastric lymphoma can occur as a part of a generalized lymphomatous process or, in 10 % of cases, could be represented by an isolated primary gastric localization [7].
The lymphomatous process generally spreads submucosally, determining an irregular diffuse inhomogeneous and hypodense smooth thickening of the gastric wall greater than 1 cm. involving more than half of the circumference of the stomach. The inner and outer margins of the lymphoma are frequently waved; the perigastric flat planes are generally obliterated by the tumor invasion. Associated diffuse bulky lymphadenopathies indicate a disseminated disease.
The perforation of gastric carcinoma and lymphoma is a rare condition with an incidence of 0.4–0.6 % [8].
It generally occurs in elderly patients with a locally ulcerated advanced tumor. The use of thin high-resolution axial CT slices can demonstrate in some cases a direct discontinuity of the gastric neoplasm. In open peritoneal perforation, an amount of fluid and free air bubbles is diffused principally around the gastric walls, in the subphrenic left space, and inside the umbilical fissure of the liver and the small omentum (Fig. 15.3).
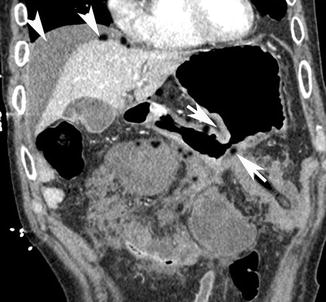

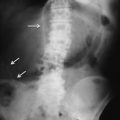
Fig. 15.3
Coronal post-contrast medium CT scan shows the narrowing of the stomach body lumen (white arrows) with secondary proximal gastrectasia. There is an open peritoneal perforation, with fluid and free air bubbles diffused around the gastric walls, inside the small omentum, and in the right subphrenic space (white arrow heads)
15.6 Small Intestinal Cancer
Tumors of the small intestine are uncommon, accounting for no more than 6 % of all gastrointestinal apparatus neoplasms, and are frequently represented by annular, aneurysmally dilated or ulcerated mass, associated with thickening or retractive desmoplastic reaction of the adjacent mesenteric fold. An endoluminal mass with a dishomogeneous post-contrast medium wall attenuation can be visualized. The evidence of ulceration is indicative of adenocarcinoma. Other CT signs are ulceration, necrotic areas, inner undulated margins, irregular outer profiles, and narrowing and obstruction of the lumen (Fig. 15.4). The perforation occurs in sites of the small intestine where an intramural infiltration by neoplastic cells has developed and has been followed by tumor necrosis. Perforation of a bowel metastasis can occur also after chemotherapy due to the cellular necrotic effect produced by the drug [9].
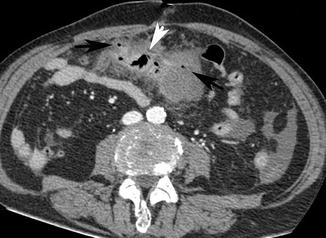

Fig. 15.4
Axial post-contrast medium CT scan shows a small bowel ulcerated mass (white arrow head), associated with narrowing of the lumen and retractive desmoplastic reaction with irregular outer profiles of the adjacent mesenteric fold in which there are multiple air bubbles (black arrows)
15.7 Small Intestinal Lymphoma
Small intestinal involvement of a lymphoma is one of the crucial points in the course of the pathology. The perforation or fistulization of this kind of localization can occur at any site including the duodenum, jejunum, and ileum [10] (Fig. 15.5).
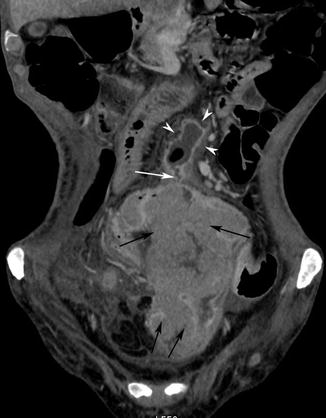

Fig. 15.5
Coronal post-contrast medium CT scan shows a very large dishomogeneous ileal mass (black arrows), associated with a thin fistula (white arrow) communicating with a mesenterial abscess (white arrow heads)
Free intestinal perforation may occur both spontaneously and after chemotherapy, and it is due to tumor necrosis (Fig. 15.6). The diagnosis is often delayed because this kind of pathology has been frequently treated with cortisone that can reduce significantly abdominal acute symptoms.
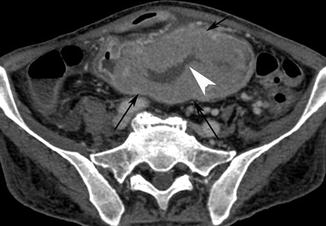

Fig. 15.6
Axial post-contrast medium CT scan shows a very large small intestinal mass (black arrows), in which there is a necrotic area (white arrow head)
If the perforation involves a small intestinal lymphoma, it is possible that in the peritoneal cavity there will be the presence only of free fluid without air. The peritoneal fluid can be evidenced by ultrasonography, but the site of the intestinal lymphoma could be better visualized by computed tomography.
The bowel wall can get ruptured due to the mechanical action of the bowel that resulted in mucosal ulceration, the depth of which increased gradually with the depth and degree of lymphoma cell infiltration, until eventually with the increase in intraluminal pressure (Fig. 15.7a, b).
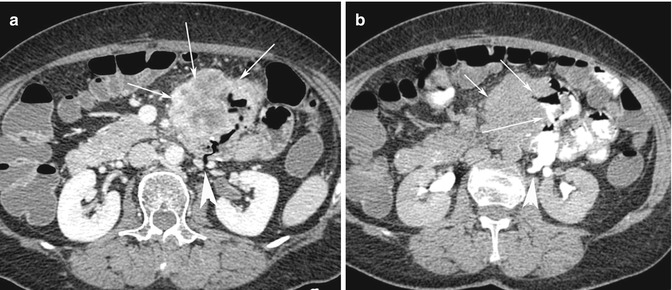

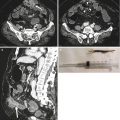
Fig. 15.7
(a) Axial post-contrast medium CT scan shows a very large dishomogeneous mass (white arrows in a and small arrow in b) of the abdominal left flank, involving an ileal loop and the adjacent mesenteric fold in which there is an ulcerated area associated with a long posterior fistula (white arrow head) communicating with the retroperitoneal space. (b) After oral administration of contrast medium, the intestinal fistula is clearly demonstrated (two white arrows), with extravasation of c.m. into the left anterior pararenal space (white arrow head)
If the lymphoma is located in the mesenteric fold, it can invade and obstruct the adjacent bowel loops leading to their perforation in the abdominal cavity (Fig. 15.8a, b) or to their fistulization in an adjacent structure, as the excretory renal system or psoas muscle [11] (Fig. 15.9a, b).
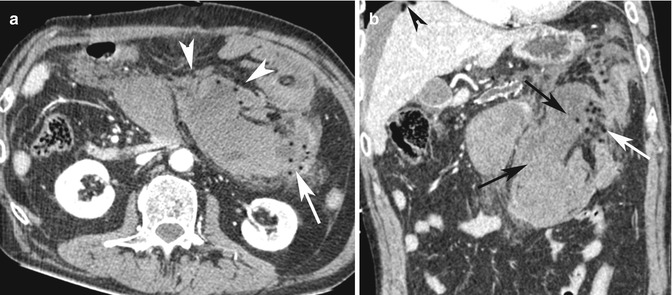
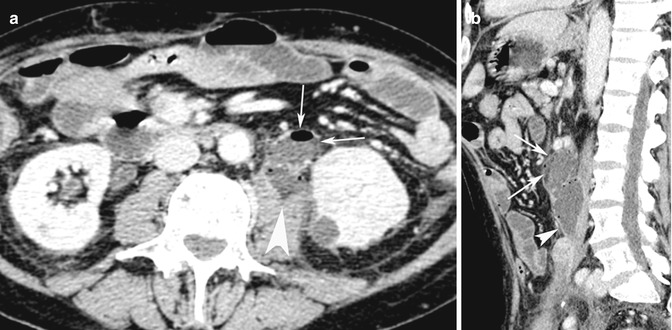

Fig. 15.8
(a) Axial post-contrast medium CT scan shows a large lymphoma located into the mesenteric fold (white arrow heads) that invades and obstructs the adjacent bowel loops (white arrow). (b) Coronal reconstruction CT image shows the involved bowel loops (black arrows) and their perforation with air bubbles into the adjacent mesenteric fold (white arrow) and into the abdominal cavity (black arrow head)

Fig. 15.9
(a) Axial post-contrast medium CT scan shows an obstructed small intestinal loop (white arrows) perforated into the psoas muscle fascia (white arrow head). (b) Sagittal reconstruction CT image better shows the perforated bowel loop (white arrows) with the development of a muscle abscess (white arrow head)
The steroid treatment itself can induce ulceration, tumor necrosis, and perforation of the intestinal bowel loop lymphoma.
15.8 Small Intestinal Metastasis
Solitary or multiple metastases spread in the small intestinal wall is much less common, although small bowel involvement in the late stage of abdominal carcinomatosis is frequently observed. When metastatic lesions of the small intestine cause symptoms, the usual presentation is that of either complete or incomplete bowel obstruction, intestinal bleeding due to ulceration and erosion, and more rarely intestinal perforation [12].
Perforation of a bowel metastasis can occur also after chemotherapy due to the cellular necrotic effect produced by the drug [9] (Fig. 15.10).
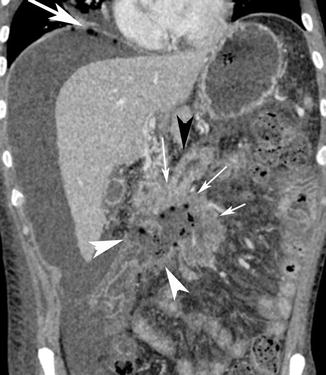

Fig. 15.10
Post-contrast medium CT coronal reconstruction shows a large mesenterial mass (thin white arrows) with a large necrotic area and irregular borders (white arrow heads) involving the duodenum (black arrow head). The necrotic mass perforation has been demonstrated by the presence of air and fluid into the peritoneal cavity (white arrow)
The most frequent histologic types that can determine hematogenous metastasis into the intestinal wall are oat cell, epidermoid, giant cell carcinomas, and melanomas. The primary tumor generally has an abdominal or pelvic cancer origin as colon, uterine, and ovarian cancer or extra-abdominal origin as pulmonary cancer, breast cancer, and melanoma [13].
15.9 Colorectal Cancer
The ascending and descending colon tumors can be readily assessed because of their fixed retroperitoneal locations. Tumors localized in the flexures and transverse colon are less frequently well evidenced by CT, for their position, retained fecal material, incomplete distension of the wall, peristaltic activity, and respiratory excursions.
The most frequent CT finding of colon cancer is represented by a focal thickening of the wall greater than 6 mm with a dishomogeneous post-contrast medium wall attenuation, frequently narrowing the intestinal lumen (Fig. 15.11).
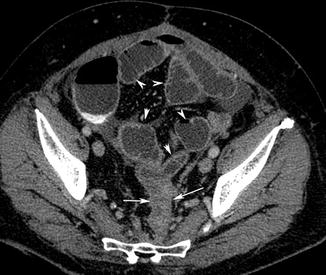

Fig. 15.11
Post contrast medium axial reconstruction shows a focal thickening of the sigmoid colon wall with a dishomogeneous post-contrast medium wall attenuation narrowing the intestinal lumen (white arrows), with mechanical obstruction of the proximal intestinal loops (white arrow heads)
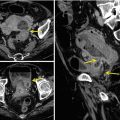
Particularly in the adenocarcinoma type, an endoluminal polypoid mass with a dishomogeneous post-contrast medium wall attenuation can be visualized. Other CT signs are ulceration, necrotic areas, inner undulated margins, irregular outer profiles, stenosis, and obstruction of the lumen (Fig. 15.12a, b).
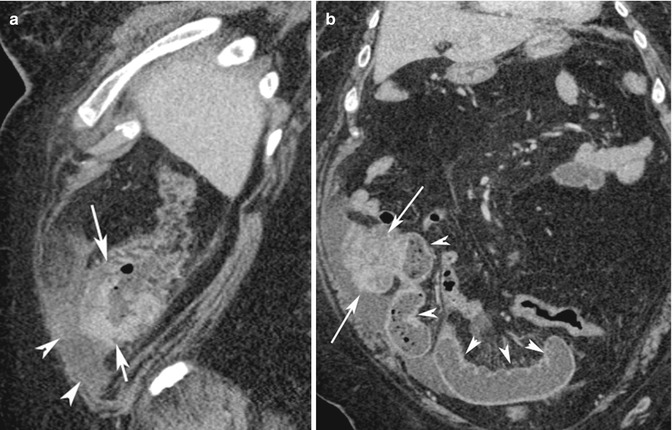

Fig. 15.12
(a) Sagittal reconstruction post-contrast medium CT image shows a neoplastic mass of the cecum pouch with inner undulated margins and irregular outer profiles (white arrows, a and b), surrounded by peritoneal fluid (white arrow heads). (b) Coronal reconstruction post-contrast medium CT image shows a mechanical obstruction of the proximal ileal loops (white arrow heads) with the lumen filled with fluid and air bubbles
The rectum and rectosigmoid tract are easily evaluated by CT because of the fixed position in the pelvic cavity.
Extracolon tumor spread is suggested by loss tissue fat planes between the large intestine and the surrounding structures as the seminal vesicles, prostate, bladder, uterus, ovaries, ureters, and muscles as the iliac, levator ani, obturator internus, piriformis, and coccygeal. Rectum and rectosigmoid tumor can invade bones as the sacrum and coccyx.
In selected cases it is possible to evidence a discontinuity of the colorectal cancer wall, with associated findings of open perforation or covered perforation (Fig. 15.13). In open perforation, an amount of dense fluid and free air bubbles is located principally in the subphrenic spaces, around the liver and the gastric walls, along the mesenteric folds, and in the peritoneal recesses of the pelvic cavity.
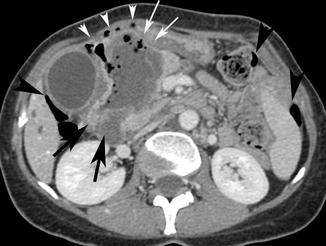

Fig. 15.13
Axial post-contrast media CT scan shows a focal thickening of the wall of the right flexure of the colon with a dishomogeneous post-contrast medium wall attenuation narrowing the intestinal lumen (black arrows). The neoplastic stenosis has been complicated both with a covered perforation due to the development of a pericolic abscess (white arrows) and open perforation with free air bubbles around the abscess wall (white arrow heads) and into the peritoneal cavity (black arrow heads)
The cover perforation with the development of an abscess has been demonstrated by a low-density unilocular or multilocular collection with air-fluid level or air bubbles (Fig. 15.14a, b), associated with an increased peripheral density of the infiltrated or inflamed surrounding fat tissue. It is generally associated with a limited amount of free peritoneal fluid mainly localized in the vesical-rectal pouch.
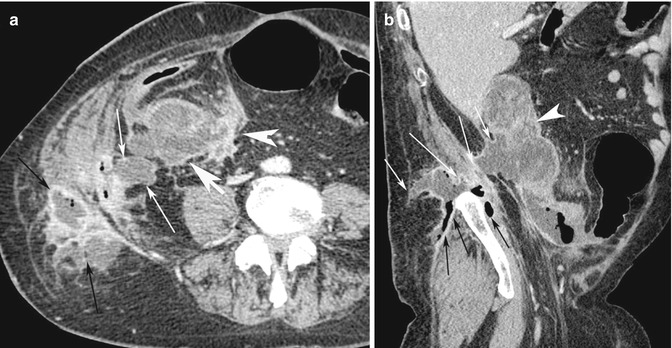

Fig. 15.14
(a) Axial post-contrast medium CT scan shows the cover perforation of the ascending colon cancer (white arrow head) with the development of an abscess, demonstrated by a low-density multilocular collection with air bubbles (white arrows), associated with inflamed surrounding fat tissue. The multiloculated abscess has been complicated with an abdominal wall fistula and development of a subcutaneous fat abscess (black arrows). (b) Sagittal reconstruction CT image shows the paracolic internal (white arrow head) and subcutaneous external abscesses (white arrows). The infection has spread also inside the muscle fasciae (black arrows)
Associated metastasis could be evidenced at the liver, adrenal glands, local lymph nodes, and peritoneal cavity. Additional findings are frequently represented by omental cake diffused distant lymph nodes.
15.10 CT Findings of Associated Intestinal Obstruction
Abnormal accumulation of gas and fluid in the distended intestinal tract occurs in the mechanical obstruction of both the small and large bowels affected by cancer (Figs. 15.11 and 15.12a, b). The distended intestinal loops contain air-fluid levels and are located proximal to the site of the neoplastic obstruction. During the acute phase of the mechanical obstruction, peristalsis become hyperactive with thickened plicae mucosae as the intestine attempts to work against the site of occlusion. When the obstruction lasts for a long time and is persistent, the intestine becomes atonic and plicae mucosae disappear.
At the level of the small intestine, the occlusion can determine an accumulation of small droplets of gas within the recesses between the valvulae conniventes, producing a series of small air bubbles referred to as the “string of pearls.”
If the site of neoplastic mechanical obstruction is the colon, a competent ileocecal valve prevents the reflux of gas and fluid in the lumen of the small intestine, resulting in a “closing loop” obstruction of the colon. The wall of the cecum, being thinner and weaker than the wall of the remainder portions of the colon, distends more rapidly and to a greater degree. Therefore the rupture of the obstructed colon most likely occurs at the cecum level.
If the ileocecal valve is incompetent, allowing gas and fluid to reflux into the lumen of the small intestine, the tear of the cecum is not probable.
The high pressure in the intestinal lumen caused by occlusion can lead to a hypoperfusion of the intestinal wall with consequent ischemia and infarction [14].
15.11 Primary Tumors and Metastasis of Small Bowel Perforation
Primary tumors and metastasis of the small bowel intestine can determine various complications as obstruction, bleeding, malabsorption, and perforation.
Mural replacement by metastatic tissue occurs after hematogenous or lymphatic spread of cancer cells. The spontaneous necrosis or the induced necrosis by chemotherapy can lead to metastasis perforation (Fig. 15.15). Also the increased luminal pressure due to the obstructed bowel loop or the embolization procedure performed for occluding the arterial supply to stop the severe bleeding of a metastasis can lead to the perforation [15].
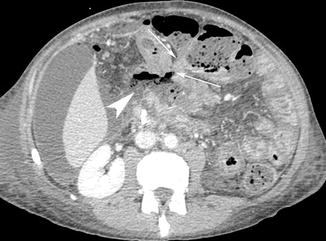

Fig. 15.15
Axial post-contrast medium CT scan shows a metastatic tissue that involves the transverse colon and adjacent ileal bowel loop (white arrows) with intestinal perforation and spread of air bubbles and enteric fluid into the mesenteric fold (white arrow head)
15.12 Gastrointestinal Stromal Tumor (GIST) Perforation
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract and are 2.5 % of all gastrointestinal tumors [16]. The stomach is the most common site accounting for 50–70 % of GISTs, the small intestine for 25–30 %, and the colon-rectum for 5 % [17]. They are well-circumscribed masses that range in size from several millimeters to 30 cm and do not have a true capsula. They are frequently inhomogeneous with hyperdense areas that depend on a significant arterial blood supply, with focal areas of hemorrhage, necrosis, calcifications, cystic degeneration, and cavitations that can communicate with the intestinal lumen, the abdominal cavity, or both. Extension into the adjacent small bowel mesentery and encasement of noncontiguous segments of the small intestine, colon, bladder, ureter, and abdominal wall can occur [18].
They involve the muscularis propria of the intestinal wall and have the propensity for outer growth, projecting into the abdominal cavity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree