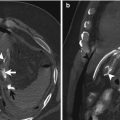
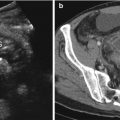
Fig. 13.1
The radiography shows both football sign, as oval-shaped peritoneal gas, and Rigler sign (arrow)
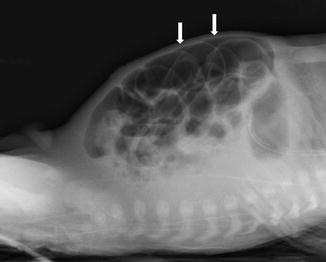
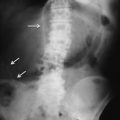
Fig. 13.2
Triangle sign: triangular gas pockets between loops of bowel (arrows) are seen in small amount of peritoneal gas
Another suggestive sign of perforation is peritoneal fluid that is associated with high mortality rate.
Gas in the intestinal wall and portal venous system in infant is invariably associated with enteritis or enterocolitis, except when the gas has been introduced accidentally through an indwelling umbilical catheter.
Upright or cross-table lateral radiograph films may show laddering air fluid levels. These findings, in conjunction with a lack of air and stool in the distal colon and rectum, are highly suggestive of mechanical intestinal obstruction (Fig. 13.3).
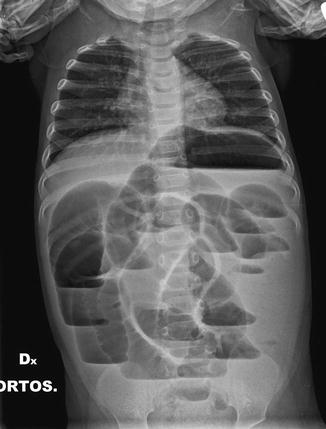

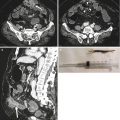
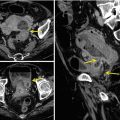
Fig. 13.3
Multiple air fluid levels and bowel parietal thickening in intussusception
In some cases of perforation of hollow viscera, the clinical and plain film findings may be inconclusive. In such circumstances, examination with contrast material may be indicated. The site of perforation may be demonstrated if extravasation of contrast medium occurs.
If a perforation is suspected, barium is contraindicated, and water-soluble agents should be used [4, 5].
Therefore, the use of Gastrografin, which contains a wetting agent, was used when looking for a gastrointestinal perforation. However, Gastrografin may cause dehydration secondary to the hyperosmolarity. Recently Iopamidol has been preferred to Gastrografin for its lower osmotic pressure, with the same rate of success.
13.2 US Examination
Ultrasound may be particularly useful in children where radiation burden should be limited. Ultrasonography could be useful as an initial diagnostic test to determine, in various cases, the presence and, sometimes, the cause of the pneumoperitoneum. Ultrasound has lower sensitivity than radiography (76 % vs. 92 %, respectively) and should be used in selected cases only (clinical conditions preventing radiographs from being performed correctly, persisting clinical suspicion with negative or questionable radiographic findings, the exclusion of other acute abdominal conditions) [6].
The main sonographic sign of perforation is free intraperitoneal air, resulting in an increased echogenicity of a peritoneal stripe associated with multiple reflection artifacts and characteristic comet-tail appearance. It is best detected using linear probes in the right upper quadrant between the anterior abdominal wall, in the prehepatic space.
Direct sign of perforation may be detectable, particularly if they are associated with other sonographic abnormalities, called indirect signs, like thickened bowel loop and air bubbles in ascitic fluid or, in a localized fluid collection, bowel thickened wall associated with decreased bowel motility or ileus.
Nevertheless, this exam has its own pitfalls. It is strongly operator dependant; some machines have low-quality images that may not able to detect intraperitoneal free air; furthermore, children are uncooperative; sonography is also difficult in patients with subcutaneous emphysema.
13.2.1 Necrotizing Enterocolitis
Necrotizing enterocolitis (NEC) is an often fatal inflammatory disease involving the intestinal tract of premature infants in which intestinal perforation is common. The condition may mimic obstruction both clinically and radiologically. NEC is considered primarily a pathology related to prematurity. Over 90 % of cases occur in preterm infants [7]. NEC can develop in any portion of the gastrointestinal tract, with the small bowel and proximal large bowel being most frequently affected [8, 9]. NEC diagnosis requires a combination of clinical, laboratory, and radiological findings.
Early diagnosis is critical to the institution of proper therapy in premature infants with necrotizing enterocolitis. In order to make the early diagnosis of NEC, plain abdominal radiographs must be taken of all premature infants with abdominal distension, vomiting, apneic spells, jaundice, or bloody stools. If the radiographs are negative, but symptoms continue, they should be repeated.
Radiological signs play an important part in making the diagnosis of NEC. [10]. Intestinal distension (Fig. 13.4) is the most frequently encountered radiological sign in patients with NEC (55–100 % of cases) [8, 11].
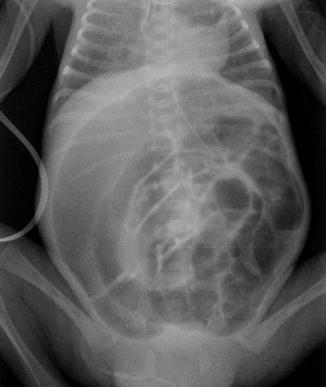

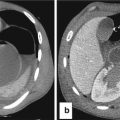
Fig. 13.4
Intestinal distension in NEC may mimic Rigler sign (see Figure), due to air inside two near intestinal loops with near bowel walls
Previous studies have reported the presence of radiological signs suggestive of peritoneal fluid in 11 % of cases and have shown a higher incidence of patient mortality in these patients. Common radiographic features included increased intraluminal gas and fluid, a “bubbly” or “frothy” appearance to the bowel, intramural gas (Fig. 13.5), hepatic portal venous gas, and pneumoperitoneum. Gas in the wall of the intestine, mesentery and retroperitoneum, and portal vein gas are the most important and useful radiographic features (Fig. 13.6). Neither is pathognomonic; both features have been observed in patients afflicted with such entities as Hirshsprung’s disease, imperforate anus, meconium ileus, small-bowel atresia, and following surgical repair of coarctation of the aorta. Portal vein gas can be introduced inadvertently through umbilical vein catheters. The sonographic appearance of free intraperitoneal air results in an increased echogenicity of a peritoneal stripe associated with multiple reflection artifacts and characteristic comet-tail appearance that can be changed by changing the patient’s position.
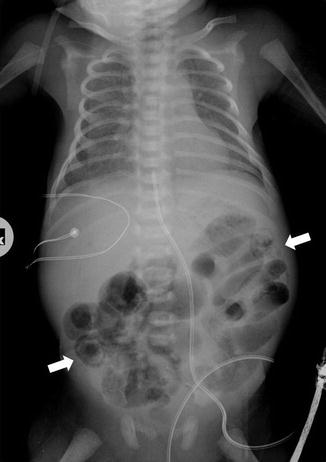
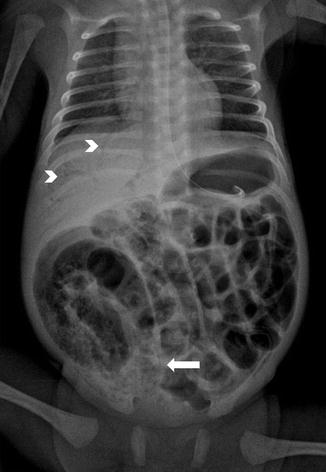

Fig. 13.5
Intramural gas (arrows) in severe pneumatosis in NEC

Fig. 13.6
Pneumatosis intestinalis, seen as lucency inside the bowel wall and bubbly appearance of bowel (arrow), and gas in the portal venous system (arrowheads) in a case of necrotizing enterocolitis
Direct sign, such as localized gas collections related to bowel perforations, may be detectable, particularly if they are associated with other sonographic abnormalities, called indirect signs (thickened bowel loop and air bubbles in ascitic fluid or, in a localized fluid collection, bowel thickened wall associated with decreased bowel motility or ileus).
13.2.2 Gastric Pneumatosis in Infancy
Gastric pneumatosis in infancy (defined as gas within the wall of the stomach) may be easily detected by radiography. It presents as a fine lucent stripe conforming to the contour of the stomach and enveloping any intraluminal gas and fluid content. The finding, though quite rare, is of almost clinical importance.
In infancy, isolated gastric pneumatosis has been seen very rarely in gastric outlet obstruction. It has also been reported in neonatal necrotizing enterocolitis, in association with intestinal pneumatosis or after perinatal stress though it may rarely be isolated.
Thus, radiographic detection of gastric pneumatosis indicates serious underlying disease, and determination of its cause will depend on the associated clinical findings.
13.2.3 Hirschsprung’s Disease
Hirschsprung’s disease is a major differential consideration in a neonate or young infant with radiographic evidence of distal bowel obstruction and clinical signs of abdominal distension, vomiting, constipation, failure to pass meconium, and failure to thrive [12–15]. Aganglionosis is the most common cause of large-bowel obstruction in the young infant [16, 17], and therefore colonic or appendiceal perforation, especially in the young infant, should raise the suspicion of Hirschsprung’s disease (Fig. 13.7). Most of the perforations reported in the literature were in the proximal colon (68 %), appendix (1 8 %), or distal small bowel (6 %). In utero perforation producing meconium peritonitis has also been described [15, 18]. The mechanism of perforation appears to be directly related to increased intraluminal pressure from distal obstruction.
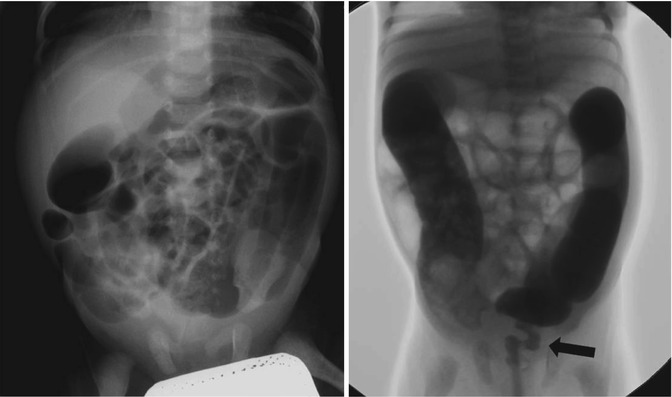

Fig. 13.7
Hirschsprung’s disease: radiography shows dilated large bowel without evidence of gas in rectum; fluoroscopic examination with contrast medium enema confirms narrowing segment of aganglionic rectum. The arrow indicates transition zone.
Long segment or total colonic aganglionosis accounted for 61 % of documented cases of Hirschsprung’s disease that presented with bowel perforation. Appendiceal and ileal perforation were particularly associated with long-segment disease.
Perforation in these cases is in aganglionic bowel, and blind colostomy at the site of perforation or in the transverse or sigmoid colon is an inappropriate treatment.
13.2.4 Meconium Ileus
Meconium abnormalities are at the origin of a series of neonatal intestinal obstructions, characterized by a wide spectrum of severity, from the benign meconium plug syndrome to the complicated meconium peritonitis and perforation. These relatively frequent and benign conditions need prompt recognition to exclude other forms of neonatal intestinal obstruction; among them meconium ileus is frequently associated to severe prematurity and low birth weight. It results from combination of highly viscid meconium in the colon or terminal ileum and poor intestinal motility, resulting in mechanical bowel obstruction.
Clinical signs of delayed meconium passage included gastric residual volumes, abdominal distension, and bilious residua. Perforated cases may be confused with NEC which is excluded by clinical history, no signs of sepsis, lab signs missing, abdominal signs missing, and typical radiological signs missing.
Management of meconium obstruction syndrome included plain radiography that reveals evidence of a mechanical obstruction and enema. Plain abdominal film shows distended small-bowel loops without air fluid levels or pneumatosis. These findings are enough to make diagnosis and exclude other forms of intestinal obstruction, mainly NEC.
Once the obstruction occurred, the risk of perforation becomes higher and is estimated around 30 %.
A softening enema with low osmotic pressure ionic X-ray contrast medium, is the first option whenever overt perforation was not present. The contrast medium leads to a propulsive hyperactive gastrointestinal motility and is diagnostic and therapeutic; however, it is not recommended for hemodynamically unstable patients [19, 20].
13.2.5 Imperforate Anus
Anorectal malformations (ARM) are common anomalies observed in neonates. The reported incidence ranges between 1:3,300 and 1:5,000 live births. They vary in severity from mild anal stenosis to complete caudal regression. These disorders usually require surgical intervention in the neonatal period and postoperative follow-up to obtain and maintain fecal and urinary continence. Diagnostic and therapeutic delays in the management of ARM may lead to complications such as sepsis, aspiration, abdominal distension, colonic perforation, respiratory embarrassment, electrolyte imbalance, and even death.
Colonic perforation due to ARM may not be avoided completely; however, early diagnosis is essential in assuring better outcomes with surgical management. Spontaneous perforation of the colon is estimated to occur in 2 % of neonates with ARM, and the incidence rises to 9.5 % when the diagnosis is delayed [23, 24].
13.2.6 Small Left Colon Syndrome
Intestinal perforation can occur as a complication of the neonatal small left colon syndrome, a condition producing signs and symptoms of low colonic obstruction.
Radiographic examination of the abdomen shows multiple dilated small-bowel loops and sometimes visualization of dilated ascending and transverse portions of the colon.
This syndrome is a benign condition in which contrast enema examination is curative by stimulating meconium evacuation. Contrast enema study demonstrates a characteristic pattern of a small left colon to the level of the splenic flexure where a sharp transition zone exists with the proximal colon being dilated. This study should be done immediately in newborns who develop clinical findings of colon obstruction or fail to pass significant meconium within 24–48 h. This aggressive approach hopefully should reduce the incidence of intestinal perforation as a complication of the neonatal small left colon syndrome. Repeat contrast enema examinations may occasionally be necessary to relieve the obstructive signs in these babies.
13.2.7 Intussusception
Intussusception occurs when a portion of the digestive tract becomes telescoped into the adjacent bowel segment. It generally occurs in children between 6 months and 2 years of age. The vast majority are ileocolic [25–27].
Intussusceptions are the second most common cause of acute intestinal obstructions in children. Once they are diagnosed, they should be treated as early as possible. Though their exact causes are not known in most of the cases, swollen Payer’s patches, enlarged lymph nodes, polyps, Meckel’s diverticulum, and duplication cysts have been suggested as few of the common etiological factors [28, 29].
The classic triad of intermittent abdominal pain or irritable crying, a palpable mass, and red currant jelly stools is reported to have a positive predictive value of 93 % for intussusceptions [30, 31].
Ultrasonography is a very useful investigation that can be used for the diagnosis. The absence of blood flow in the lesion on color Doppler study correlates significantly with the high incidence of the complications and irreducibility [28, 29, 32].
On the ultrasound image the intussusceptions is a complex structure due to the amount of mesentery [33]. The intussusceptions (the receiving loop) contain the folded intussuscepted (the donor loop), which has two components: the entering limb and returning limbs. Ultrasound obtained at the apex shows a hyperechoic outer ring separated from a hypoechoic center by a thin hyper echoic ring, which likely represents the opposed serous surface of the intussuscepted. Ultrasound obtained near the apex shows multiple concentric rings with a hypoechoic ring surrounding a hyperechoic ring, which surrounds another hypoechoic ring. US scan obtained at the base shows the central limb of the intussusception eccentrically surrounded by the hyperechoic mesentery that show the crescent in doughnut sign.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree