Key Facts
- •
Infections of the bones and joints result from one of three mechanisms of spread: (1) hematogenous, (2) contiguous, or (3) direct implantation.
- •
Radiographic hallmarks of osteomyelitis are cortical and medullary destructive osteolysis with soft tissue swelling in the acute phase and reactive sclerosis and periosteal reaction in the subacute to chronic phase; sequestrum and involucrum formation with sinus tracts differentiate chronic active from background chronic inactive osteomyelitis.
- •
Pediatric osteomyelitis occurs most commonly in the metaphysis of a long bone, usually around the knee, and is often multifocal. Adult osteomyelitis occurs most commonly in the diaphysis of a long bone.
- •
Staphylococcus aureus is the most common organism regardless of age.
- •
Brodie’s abscess, or focal subacute osteomyelitis, is a walled-off infection with no sequestrum. It has a four-layered target appearance on contrast-enhanced magnetic resonance imaging (MRI). It can be differentiated from an osteoid osteoma with confidence when a sinus tract is identified to the growth plate. Bone abscess should be considered for any destructive metaphyseal lesion extending into the epiphysis.
- •
MRI hallmarks of spine infection are disk space narrowing, vertebral end plate destruction/loss of sharp disk outline, and paraspinal mass.
- •
Radiographic hallmarks of septic arthritis are periarticular osteoporosis, joint effusion, and soft tissue swelling in the early phase, with cartilage destruction and subchondral plate destruction in the later phase.
- •
Tuberculous arthritis is characterized by the Phemister triad of periarticular osteoporosis, peripheral erosions, and absence of joint space narrowing. Although similar in appearance to rheumatoid arthritis, when involvement is monoarticular and erosions are poorly defined, tuberculosis should be considered.
- •
Immunocompromised patients are prone to aggressive soft tissue infection. Diabetic patients are prone to foot infection.
- •
Technetium-99m three-phase bone scan, although nonspecific, is useful to differentiate soft tissue infection from joint or bone infection, especially in children. To improve specificity for infection, bone scan may be combined with gallium 67 citrate or indium 111 labeled white blood cell scanning.
- •
Percutaneous aspiration or biopsy should be used to confirm infection and obtain an organism for treatment planning.
Imaging studies are important adjuncts to clinical history, tissue cultures, and laboratory tests in patients with suspected musculoskeletal infection. Once the diagnosis is considered, appropriate imaging studies should always include radiographs, with ultrasound, magnetic resonance imaging (MRI), computed tomography (CT), or radionuclide scans selectively used to help confirm diagnosis, determine extent of involvement, and facilitate pathologic diagnosis through biopsy or aspiration.
In this chapter we will divide musculoskeletal infections first by their anatomic involvement—the bones, joints, or soft tissues with special attention to infection in the spine and foot. Then we will consider the interplay of acuity of infection, route of infectious spread, organisms involved, and host age and immune status. The imaging features that characterize each type of infection will be explored by modality, identifying the benefits and drawbacks of each technique.
ADULT OSTEOMYELITIS
Infection involving the bone is termed osteomyelitis . Bone is at risk for such infection when its integrity is compromised by trauma, a large inoculum, or foreign body. The ensuing infection may then resolve or become chronic. Rather than being an inevitable stepwise progression from one entity to the next, multiple factors come into play to determine whether acute osteomyelitis will progress, including host factors (immunocompromised status, comorbidities) and treatment instituted (whether sufficiently early and appropriate). These are important for long-term prognosis, as the sequelae of these infections become graver with progression of disease. In the appropriate clinical context, when osteomyelitis is suspected, imaging is a valuable adjunct to laboratory findings for early confirmation, which in turn will facilitate the early treatment necessary to improve outcome. Complications of delayed treatment include pathologic fracture, growth disturbance, and, infrequently, malignancy in a chronically draining sinus tract.
There are three clinical manifestations of osteomyelitis based on acuity of infection ( Table 19-1 ):
- 1
Acute: Begins with marrow edema, cellular infiltration, hyperemia, and possibly microabscess.
- 2
Subacute: Increased pressure spreads infection from the marrow to the cortex via haversian and Volkmann’s canals, extending subperiosteally, eventually through the periosteum and into the soft tissues or joint. Local hyperemia and inflammation cause osteolysis and surrounding soft tissue degradation. Cortical compromise can lead to fracture, slipped epiphysis, premature growth plate closure, and chronic infection. Subacute infection may present with fever, pain, and periosteal elevation. Brodie’s abscess, or focal subacute osteomyelitis, often presents with long-standing dull pain without fever.
- 3
Chronic: Defined by symptoms persistent for more than 1 month before therapy or following inadequate treatment of acute osteomyelitis; chronic osteomyelitis is characterized clinically by drainage at the site of infection and low-grade fever. The hallmark in adults is a necrotic sequestrum, the result of ischemic necrosis caused by increased intramedullary intraosseous pressure.
A sequestrum is the hallmark of chronic active osteomyelitis and consists of a fragment of devitalized bone.
The sequestrum may be surrounded by granulation tissue or living reactive new bone (i.e., involucrum), often within the thickened cortex. The sequestrum may be extruded to the skin surface through the involucrum via a cloaca (sinus tract).
Osteomyelitis can spread by three routes ( Table 19-2 ):
- 1
Hematogenous: Hematogenous infection is most commonly caused by Staphylococcus aureus. In adults, acute hematogenous osteomyelitis usually begins in the diaphyses; infection can spread throughout the medullary canal to include the epiphysis and joint. The periosteum is tightly attached in adults, resulting in less periosteal elevation than is seen in children. Cortical breakthrough usually leads to soft tissue abscess, and sinus tracts develop from sequestrations to the skin; in children, who have the periosteum more loosely adherent to bone, subperiosteal abscess rather than soft tissue abscess results. Intramedullary abscess most commonly occurs as a single lesion near the metaphysis of the distal tibia.
- 2
Contiguous: This type of spread occurs most commonly in adults, particularly in the diabetic foot, from adjacent soft tissue or decubitus ulcers.
- 3
Direct implantation, posttraumatic or postoperative: Organisms are introduced through acute inoculation into bone at the time of trauma or spread from adjacent soft tissue infection. This category includes infections from interventions. As opposed to hematogenous spread, infections due to direct implantation are usually caused by multiple organisms including S. aureus and coagulase-negative staphylococci 75% of the time, as well as gram-negative bacilli and anaerobic organisms.
| Method of Spread | Cause | Usual Organisms |
|---|---|---|
| Hematogenous | Bloodborne infection | S. aureus |
| Contiguous | From adjacent ulcer | Multiple organisms |
| Direct implantation | Posttraumatic or postoperative | Multiple organisms |
Imaging Features
Radiographs
Acute Osteomyelitis
Within 1 to 2 days postinfection there is soft tissue swelling with loss of fascial planes.
Swelling in osteomyelitis obliterates fascial planes, whereas tumors usually displace facial planes.
Focal osteopenia may be noted.
By 7 to 10 days there is destructive bony lysis, but radiographs can be normal for 10 to 21 days postinfection, as 30% to 50% loss of medullary bone is required to see changes on radiographs.
Radiographs are insensitive for early osteomyelitis because bone changes may be inapparent for several weeks after infection.
Bone changes in early osteomyelitis therefore lag about 2 weeks behind the infection itself, and following treatment, radiographic improvement generally lags behind clinical improvement.
Following treatment, radiographic improvement generally lags behind clinical improvement.
The sensitivity of radiographs ranges from 43% to 75% and the specificity from 75% to 83% for evidence of osteomyelitis. Early radiographs are useful, however, for evaluating alternate diagnoses such as neoplasm, injury, or osteoarthritis.
By 2 to 6 weeks, radiographs generally show destruction of cortical and medullary bone, periosteal reaction, and new bone formation ( Figure 19-1, A ).


Subacute osteomyelitis
Subacute osteomyelitis can occur in the setting of inadequate treatment or when the underlying bone is abnormal. The cortex becomes more lucent as infection spreads into cortical haversian and Volkmann’s canals. Although uncommon in adults, subperiosteal abscess is evidenced by periostitis with subperiosteal new bone and involucrum formation. Periosteal disruption with adjacent soft tissue abscess is evidenced by soft tissue swelling and mass-like opacity obliterating fat planes, with bony disruption commonly leading to pathologic fracture in adults.
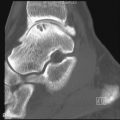
Focal subacute osteomyelitis (Brodie’s abscess)
Radiographs of a patient with a Brodie’s abscess show a sharply defined radiolucent lesion with a sclerotic margin that fades peripherally such that the outer border appears fuzzy. These lesions can also appear on imaging studies as elongated serpiginous lucencies. There is no associated sequestrum. Brodie’s abscess occurs most frequently at the metaphysis of long tubular bones, often the tibia or femur ( Table 19-1 ).
Brodie’s abscess is a focus of subacute osteomyelitis that is usually in the metaphysis of a long bone.
Chronic osteomyelitis
By 6 to 8 weeks, signs of chronic active osteomyelitis appear after inadequate treatment or treatment in immunocompromised patients. A sequestrum (necrotic bone) is formed as the blood supply to the metaphyseal and periosteal vessels is disrupted by infectious thrombi. The sequestrum is surrounded by an involucrum (periosteal new bone), which forms when pus penetrates the cortex, stimulating periosteal new bone formation. Sinus tracts form in the soft tissues relatively frequently in adults, which may extrude sequestra that then migrate to the skin surface ( Figure 19-1, B ). The cortex is thickened with the bone having an expanded appearance. Garre’s sclerosing osteomyelitis is a type of chronic osteomyelitis characterized by marked cortical thickening or periosteal reaction producing a very dense appearance on radiographs.
Sequestration is a hallmark of chronic active osteomyelitis, and adequate treatment requires surgical removal of this necrotic bone nidus ( Figure 19-2 ).

The presence of a sequestrum, which can best be documented by CT, indicates chronic active osteomyelitis. It requires surgical removal.
Sensitivity of radiographs for identifying sequestration in chronic active osteomyelitis has been reported at 9% to 33%.
Classic radiographic features of chronic active osteomyelitis thus include the following ( Table 19-3 ):
- •
Destruction of medullary bone with mottled lucencies ( Figure 19-3, A, B )




FIGURE 19-3
Osteomyelitis. This 44-year-old woman with Charcot-Marie-Tooth polyneuropathy was status post tibiotalar, talonavicular, and calcaneocuboid fusion. Internal fixation hardware was replaced with external fixator due to nonunion. MRSA positive wound infection with tibial osteomyelitis then complicated external fixation necessitating its removal. (A) AP radiograph before and (B) after removal of most of the fixation hardware demonstrate prominent lucency centered at the lateral aspect of the tibiotalar joint (arrows) , and prominent periosteal reaction of the distal tibia (open arrow) . The distal fibula has been resected in B. C, Coronal reformatted CT demonstrates periosteal reaction (arrowheads) and lucency along the distal tibia with adjacent soft tissue swelling (arrows) consistent with osteomyelitis. Subcutaneous swelling is indicated by replacement of the subcutaneous fat tissue by fluid density. C, Calcaneus; T, tibia. D, Coronal T1-weighted fat-suppressed MRI after intravenous gadolinium demonstrates contrast enhancement within the distal tibia ( arrows ) consistent with osteomyelitis, with marked enhancement in the lateral soft tissues.
- •
Periosteal new bone formation ( Figure 19-4, A )





FIGURE 19-4
Osteomyelitis with intraosseous gas and positive bone scan. 51-year-old man status post removal of fixation hardware for distal tibial and fibular fractures. A, Radiograph demonstrates cortical thickening with well-organized periosteal reaction along the distal tibial fracture site. B, CT demonstrates destructive lesion (arrow) in the intramedullary tibial plafond with intraosseous gas (arrowhead) , consistent with active osteomyelitis. The wound culture grew Streptococcus . C, CT scan shows small dense fragments, sequestra ( arrows ). D, Bone scan demonstrates increased uptake on the initial blood pool image ( arrow ). E, Delayed bone scan image shows increased uptake at the site of osteomyelitis.
- •
Cortical thickening and reactive sclerosis ( Figure 19-5, A )



FIGURE 19-5
Chronic osteomyelitis. 84-year-old man with World War II–era gunshot wound to the knee, with a 60-year history of intermittent drainage from the distal femur. A, Oblique radiograph demonstrates knee fusion with cortical thickening and periosteal reaction of the distal femur. Multiple metal fragments are noted in the anterior soft tissues. B, T1-weighted MRI after intravenous gadolinium demonstrates a fluid signal area in the distal femoral metadiaphysis with a peripheral rim sign (arrow) and two sinus tracts (arrowheads) to the skin, consistent with the patient’s penicillin-sensitive S. aureus –positive chronic active osteomyelitis. The internal low signal areas are consistent with foreign material. Note the site of soft tissue scarring (open arrow) that was evident on the radiograph. C, Bone scan showing mild delayed uptake in the distal femoral metadiaphysis (arrow) .
- •
Sequestration ( Table 19-3 )
- •
Soft tissue calcification suggesting a sinus tract
Computed Tomography (CT)
Acute osteomyelitis
In early osteomyelitis, CT can be a useful adjunct to radiographs, as it can be more sensitive for detection of cortical destruction and new bone formation, periosteal reaction and soft tissue involvement, as well as medullary increased attenuation, and medullary cavity constriction. Intraosseous gas is an uncommon but pathognomonic manifestation of osteomyelitis.
In acute osteomyelitis, MRI is preferred to CT. In chronic osteomyelitis, CT is helpful in documenting a sequestrum and will complement MRI findings.
Drawbacks to CT include lower specificity of findings such as increased medullary density, which can be seen in hemorrhage, neoplasm, stress fracture, and postradiation change. Scatter metallic artifact can degrade the images and therefore be a limiting factor.
Serial CT is specifically considered the optimal imaging technique to distinguish cranial osteomyelitis from soft tissue infection. It is useful for monitoring of the response to therapy in elderly diabetic patients who may present with a rare but potentially lethal Pseudomonas infection of necrotizing external otitis.
Chronic osteomyelitis
To differentiate chronic active osteomyelitis from a background of chronic inactive osteomyelitis requires careful evaluation for irregular foci of destructive osteolysis, fluffy periostitis ( Figure 19-3, C ), soft tissue swelling, sequestra, and sinus tracts, which are all signs of an active infection. CT is particularly useful to visualize small sequestra in areas of bony sclerosis, periosteal reaction, cortical erosion, foreign bodies, and subtle sinus tracts in bone. CT can be better than MRI to identify marrow calcification and cortical destruction. Tumeh et al. found CT 100% sensitive for sequestra, but the examination can be falsely positive due to bone remodeling and sclerosis from old healed infections.
Nonspecific CT findings of inflammation include medullary hyperattenuation, which can be seen with a differential diagnosis of hemorrhage, neoplasm, fracture, and radiation. Increased specificity is seen with cortical bone destruction, new bone formation, decrease in size of the medullary space, sequestration, and intraosseous gas ( Figure 19-4, B, C ). Localization of the sequestrum is important, as this needs to be surgically removed to adequately treat the infection or else it will continue to serve as a nidus of active osteomyelitis.
Magnetic Resonance Imaging
Acute osteomyelitis
MRI is the most useful modality for early diagnosis and determination of extent of infection, offering better marrow and soft tissue evaluation than CT and greater anatomic detail than nuclear medicine examination.
MRI is the method of choice for imaging acute osteomyelitis. Intravenous contrast is indicated when evaluating possible osteomyelitis. Radiographs should also be obtained in most cases prior to MRI to exclude other diagnoses and for correlation with MRI findings.
On T1-weighted spin echo imaging, fatty bone marrow normally shows bright signal, cortical bone shows dark signal, and cartilage shows intermediate signal. Marrow replacement by higher-water-content material may be due to a wide variety of conditions such as neuropathic osteoarthropathy, tumor, infarct, ischemia, fracture, or infection. In all of these conditions, the marrow fat will be replaced and the marrow will appear darker on T1-weighted images and brighter on T2-weighted images. A greater degree of T2 signal intensity does, however, seem to correlate with a greater likelihood of osteomyelitis. Often the pattern of the marrow changes or other findings will help identify the presence of infection. These supporting secondary MRI signs for osteomyelitis include: soft tissue ulcer, cellulitis, soft tissue abscess, sinus tract, and cortical interruption. MRI examination may be limited to various degrees by artifact from metallic prostheses, and the ability of the patient to tolerate the examination due to their hemodynamic status or claustrophobia.
Modic et al. also report that some culture positive cases of osteomyelitis show low signal on T1-weighted and T2-weighted imaging, with similar signal characteristics to fibrous dysplasia, bone infarction, and benign sclerosis. In the presence of metallic prostheses, the degree of artifact limits the utility of MRI, making indium-labeled white blood cell (WBC) scanning generally more sensitive and specific in this scenario, as well as in the immediate postoperative or posttraumatic period.
When metallic prostheses are present, indium-labeled WBC scanning may be more helpful than MRI because of the artifacts associated with prostheses on MRI examination.
MRI is more useful than nuclear imaging for differentiating soft tissue infection with periostitis from osteomyelitis.
MRI is the most useful modality to identify or exclude osteomyelitis when soft tissue infection and periosteal reaction are present.
The sensitivity of MRI (using fat-suppression technique) for osteomyelitis is variable, generally ranging from 82% to 100%, with specificity ranging from 75% to 100%, which is greater than radiographs or CT and comparable to radionuclide studies. However, sensitivity has been reported as low as 60% and specificity as low as 50%. Short tau inversion recovery (STIR) sequences are highly sensitive but less specific than spin echo sequences, with negative predictive value of STIR for acute osteomyelitis reportedly near 100% but with limited spatial resolution that cannot reliably differentiate abscess from edema.
A normal STIR sequence on MRI essentially rules out osteomyelitis.
Pitfalls of MRI for diagnosing osteomyelitis include evaluation for marrow abnormalities in anemic patients and in other marrow replacement abnormalities, with diagnosis particularly difficult in small and flat bones.
Focal subacute osteomyelitis
T1-weighted post-gadolinium images of Brodie’s abscess characteristically show a “target” lesion with four layers, the central abscess being low signal, surrounded by enhancing intermediate signal granulation tissue lining the inner wall (“double line” sign) of the abscess, surrounded by a sharp low signal “rim sign” of fibrosis, and, most peripherally, higher signal endosteal reaction/hyperemia.
Brodie’s abscess typically shows a target lesion with four layers on T1-weighted MRI.
Chronic osteomyelitis
In chronic osteomyelitis, tissue planes become more sharply demarcated. Intraosseous edema also becomes more sharply demarcated with a peripheral “rim sign” of low T1 and T2 signal due to devascularized fibrosis. This gain is reportedly seen in 93% of patients with posttraumatic chronic osteomyelitis. T2 bright sinus tracts may be present ( Figure 19-5, B ).
In the difficult clinical quandary of diagnosing reactivation of osteomyelitis in a site of chronic posttraumatic osteomyelitis, MRI has a reported sensitivity of 100%, specificity of 60%, and accuracy of 79%, with negative predictive value of 100%. Sequestra, cloacae, and soft tissue and subperiosteal abscesses should suggest an active component.
A negative MRI is highly reliable in ruling out active osteomyelitis in a patient with chronic posttraumatic osteomyelitis.
Higher signal at T1-weighted images signal can suggest healed infection with fatty marrow replacement rather than active infection. Sequestra tend to be low signal on all sequences and nonenhancing when derived from cortical bone but higher signal intensity when derived from cancellous bone. Soft tissue abscesses are well-demarcated T2 bright foci. Gadolinium administration allows for high sensitivity but cannot reliably differentiate infection (see Figure 19-3, D ) from adjacent joint inflammation, trauma, osteoarthritis, diabetic osteoarthropathy, neoplasm, or radiation ( Figure 19-6, A and B ). Lack of enhancement, on the other hand, offers good support against infection. Gadolinium can help differentiate chronic from acute processes, with infarcted sequestra showing no enhancement, and can help differentiate abscesses from tumor, as abscesses do not enhance.


Complications of longstanding chronic osteomyelitis include squamous cell carcinoma development within a sinus tract in 0.23% to 1.6% of such patients. This can be identified by the presence of new lytic bone lesions on radiographs or by a soft tissue mass on MRI.
MRI can aid in surgical planning to determine extent of disease, involvement of critical structures, and the presence of devitalized tissue.
Scintigraphy
Nuclear medicine provides physiologic information regarding inflammation, but with lower anatomic resolution than MRI. Three radionuclide classes are most commonly used: (1) technetium-99m (Tc-99m) MDP bone scan, (2) gallium-67 (Ga-67) citrate, and (3) indium-111 (In-111) labeled or Tc-99m hexamethylpropyleneamine oxime (HMPAO) labeled WBCs. The accuracy of the bone scan is increased by combining it with gallium-labeled or indium-labeled WBC scanning. A number of additional radiopharmaceuticals, however, are being evaluated for improved accuracy. This topic is explored in greater depth in the nuclear medicine section, Chapter 2 .
Acute osteomyelitis
Three-phase bone scan is a routine technique used to distinguish cellulitis from osteomyelitis; the former shows increased uptake on the first two phases of the bone scan but not on delayed imaging, and the latter shows increased uptake on all phases within 1 to 3 days of symptom development. Bone scan has high sensitivity but low specificity in diagnosing osteomyelitis, with a sensitivity ranging from 69% to 100% and specificity ranging from 38% to 82%. The relatively low specificity can be attributed to numerous diagnoses resulting in increased bone turnover, including infection, neoplasm, and trauma, that show a similar appearance by this technique. In postoperative, posttraumatic, or neuropathic patients, infection may therefore be difficult to differentiate from the underlying disease. Bone scan does offer the opportunity to detect multiple sites of infection simultaneously. Three-phase whole-body bone imaging, developed by Yang et al. in 1988, has also been used to differentiate between cellulitis and sites of active and inactive osteomyelitis.
Bone scan followed by Ga-67 scan, which tends to be more specific, shows increased uptake with inflammation and decreased uptake in response to therapy. In-111 labeled WBC imaging has both a high sensitivity and specificity for diagnosing acute osteomyelitis, especially in the extremities, with a reported sensitivity of 83%, specificity of 94%, and accuracy of 88% for osteomyelitis.
Three phase bone scan can distinguish cellulotis from osteomyelitis.
Chronic osteomyelitis
Any form of osteomyelitis can progress to a chronic form involving necrotic bone. Three-phase bone scan remains useful to distinguish cellulitis from osteomyelitis (see Figure 19-4, D , Figure 19-4, E , and Figure 19-5, B ). Combined bone scan and leukocyte scan is limited in the setting of low-grade chronic infections (see Figure 19-5, C ), in closely adherent soft tissue infection, in the central skeleton where there is a predominance of hematopoietic marrow, and posttraumatic or postsurgical cases that have ectopic hematopoietic marrow. Combined bone scan and gallium scan are the current radionuclide gold standard for vertebral infection. To diagnose chronic active osteomyelitis, Tc-99m sulfur colloid marrow imaging combined with In-111–labeled WBC imaging has been advocated. Tumeh et al. found scintigraphy to be a helpful adjunct imaging technique in excluding the presence of active disease when the CT is falsely positive for sequestra, hence avoiding surgery.
Positron Emission Tomography
As an alternative to combined nuclear medicine scanning, 18-F-fluoro-D-deoxyglucose positron emission tomography, or FDG-PET, has shown particularly promising results in evaluating patients with chronic osteomyelitis. These images are not compromised by metallic implants and can differentiate between scar tissue and active inflammation. It is of particular utility for its greater accuracy in the axial skeleton, where alternate nuclear imaging techniques are compromised. It may become useful for more specific follow-up of therapeutic response to antibiotics, as it quantitatively images activated inflammatory cells rather than the hyperemia that is imaged with bone scan, CT, and MRI. Stumpe et al. evaluated 45 whole- or partial-body FDG-PET examinations to detect soft tissue and bone infections. Sensitivity of PET was found to be 96% for soft tissue infections and 100% for bone infections. Specificity for this group ranged from 70% to 99%.
PET scanning may be a method for accurately identifying and following acute infection.
Ultrasonography
Ultrasound is easily accessible, inexpensive, without ionizing radiation, and allows dynamic real time examination to guide biopsy, aspiration, or drainage. The utility in evaluation of osteomyelitis is in the evaluation for subperiosteal fluid collections, which are diagnostic in the appropriate clinical setting. Changes highly suspicious for osteomyelitis include abscess or fluid collections next to the cortex. Ultrasound evaluation is not hampered by metallic prostheses, as is the case with cross-sectional modalities.
ADULT SPINAL INFECTION
Infection in the spine can involve several anatomic areas and is usually spread by the hematogenous route but can also be spread by contiguous infection or direct implantation. Areas involved can include the vertebral body, the disk, the paravertebral soft tissues, or the epidural space.
Infection beginning in the anterior inferior body suggests spread from the anterior spinal artery. Segmental vertebral arteries usually bifurcate to supply two adjacent vertebrae, and infection usually involves these vertebrae and their intervening disk.
Typically, infection involves two adjacent vertebrae and the intervening intervertebral disk. This is distinct from tumor involvement that usually spares the disk.
Of hematogenous vertebral osteomyelitis, approximately 50% of cases involve lumbar vertebrae, 30% involve thoracic vertebrae, and 20% involve cervical vertebrae. Forty percent of adult vertebral osteomyelitis is reportedly associated with retrograde urinary tract infection (UTI) spread through Batson’s venous plexus. Other causes are IV drug use, endocarditis, diabetes, and sickle cell disease. In IV drug use, the incidence of cervical vertebral involvement increases (27%) and thoracic vertebral involvement decreases (4.5%).
Vertebral osteomyelitis may quickly spread to adjacent vertebrae and cause discitis, epidural and subdural abscesses, meningitis, paravertebral, retropharyngeal, mediastinal, subphrenic, or retroperitoneal abscesses.
The most common organism involved is S. aureus in normal patients (accounts for 90% of spine infections), or aerobic gram-negative rods including Pseudomonas aeruginosa and Serratia marcescens in IV drug users and patients with urinary tract infections. Granulomatous infection is also seen including tuberculosis (TB), brucellosis, or coccidioidomycosis.
Imaging Features
Radiographs
Disk space narrowing and vertebral endplate irregularity are seen with pyogenic spondylodiscitis, which can progress to vertebral collapse ( Figure 19-7, A ).






Tuberculous spondylitis, or Pott’s disease, can involve the vertebrae or disk, and the lower thoracic and upper lumbar vertebrae are affected 25% to 50% of the time. Brucellosis, in comparison, often involves the lower lumbar spine and maintains vertebral bodies intact with the disk spaces decreased, posterior elements not affected, and epidural and paraspinal spaces less affected than with TB. In disseminated coccidioidomycosis, the granulomatous infection of the southwestern United States, the spine can be involved 10% to 50% of the time with multiple foci throughout the vertebrae but with disk spaces preserved.
Computed Tomography
Vertebral osteomyelitis can be evaluated with contrast enhanced CT, which will show end plate erosions, paravertebral masses, and hyperemic disk enhancement ( Figure 19-7, B ). If the soft tissue mass extends around most of the vertebral body, infection rather than tumor is likely.
Magnetic Resonance Imaging
MRI has proven to be the modality of choice to evaluate spine infection, as it shows vertebral infection with a sensitivity of 96% and specificity of 93% and allows delineation of epidural and paraspinal abscesses.
MRI with gadolinium is the modality of choice for evaluating possible spine infection.
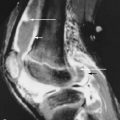
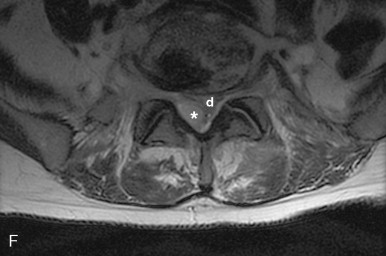
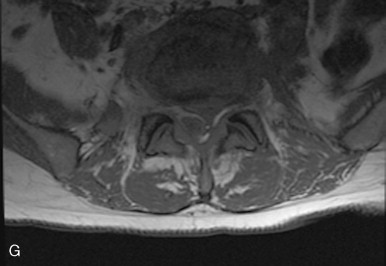
In the spine, vertebral osteomyelitis is characterized by disk height loss, confluent low T1 signal in the vertebrae and disks with blurring of the end plate margins, and high T2 signal and enhancement within the vertebrae and usually within the disk ( Figure 19-7, C-E ). On T2-weighted imaging, normally the disk is high signal bisected by a thin dark line, or cleft, which is thought to be fibrous tissue and is seen normally by age 30. Changes within the disk with infection include obliteration or irregularity of this cleft. Epidural abscesses are well-defined, usually T1 isointense, T2 hyperintense foci, but can show signal heterogeneity and partial or diffuse enhancement with gadolinium ( Figure 19-7, F-H ).
MRI can be more useful than radiography or nuclear scintigraphy in differentiating degenerative or neoplastic involvement from infectious involvement. Degenerative disks are distinct from the vertebral end plates on T1-weighted imaging and show low T2 signal, as opposed to high T2 signal in infection or neoplasm. Neoplasm does not show abnormality crossing the disk space on both the T1-weighted and T2-weighted imaging as occurs with active osteomyelitis that has progressed to disk involvement.
In tuberculous spondylitis, findings include disk space narrowing and destruction of the adjacent endplates, often with a paraspinal psoas abscess. Complications include most commonly spinal stenosis with paraplegia, but vertebral collapse with kyphosis and gibbus formation can also occur, with MRI being useful to evaluate any cord compromise. When coccidioidomycosis involves the spine, it rarely involves the disk, can present with paraspinal soft tissue extension, and does not tend to cause the gibbus deformity seen with TB.
Following treatment, MRI signal can remain abnormal for 6 weeks to 1 year. The long duration of healing change is likely related to fibrous scar tissue and new bone formation.
Scintigraphy
Gallium scanning is useful for diagnosis of vertebral osteomyelitis and can be more accurate than MRI in evaluating for isolated epidural abscess, as the signal can be obscured on MRI by the adjacent similar cerebrospinal fluid signal. In the presence of antibiotics, gallium scans may return to normal, which can be an indicator of clinical response.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree