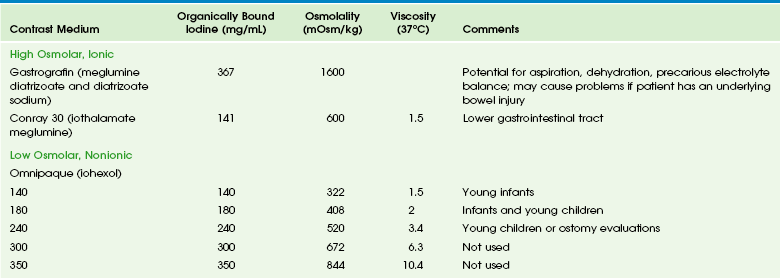
Chapter 85 The mainstay of further assessment of suspected pathology of the hollow viscera remains fluoroscopy, although sonography, computed tomography (CT), scintigraphy, and, increasingly, magnetic resonance imaging (MRI) also are applicable and will be discussed later in this chapter. To limit the radiation dose, fluoroscopy should be intermittent; pulsed fluoroscopic techniques can decrease the radiation dose substantially without loss of clinical information.1,2 Capture and storage of the fluoroscopic images can be used liberally to document such findings as viscus distension, course of contrast, and peristaltic activity, with spot films reserved for areas in which greater anatomic detail is of diagnostic importance, such as mucosal abnormalities and potential perforation with contrast leaks. Fluoroscopic studies typically require use of enteric contrast material for diagnosis.3,4 Barium, an inert substance that is not absorbed, remains the primary contrast medium used in fluoroscopic procedures, whether it is administered orally to evaluate the esophagus and upper GI tract or rectally in a contrast enema. Several barium preparations are available; barium sulfate powder (96% wt/wt) can be diluted with sterile water for infant upper GI examinations to the desired concentration of 40% to 60% wt/vol. Premixed suspensions (60% wt/vol) can be used in older children, adolescents, and adults. Enema kits containing 97% barium wt/wt can be mixed with water to a final concentration of 15% to 33% barium wt/vol for infants, older children, and adolescents. Although adverse reactions to barium products are rare—reportedly 2 per million or less5—they do occur and may present as a rash, loss of consciousness, and anaphylaxis, typically related to any one of several additives, such as methylparaben and carboxymethylcellulose.5–7 Aspiration of barium in small quantities is tolerated, but aspiration of a large volume of barium can be fatal.8,9 Barium is contraindicated in cases in which viscus perforation is suspected. In such cases, a low-osmolality, nonionic, water-soluble iodinated medium such as iohexol is used (Table 85-1). It is important that hypertonic media such as ionic or high-osmolality media (e.g., diatrizoate or iothalamate) not be used orally because of the risk of aspiration and consequent pulmonary edema.10–12 Table 85-1 Commonly Used Types of Contrast Media in Fluoroscopy of the Gastrointestinal Tract Data from package inserts. Gastrografin (diatrizoate meglumine and diatrizoate sodium) is an ionic, markedly hypertonic iodine solution with an osmolality (mOsm/kg) of approximately 1600; a 1 : 5 dilution approximates serum osmolality (285) but also dilutes the iodine concentration. Ionic hyperosmolar media can be absorbed from the GI tract and thus pose a risk in patients with a history of hypersensitivity, particularly to iodine, and potentially in patients with thyroid disease. Hyperosmolar media cause severe pulmonary complications of edema and pneumonitis if aspirated and can cause major fluid shifts into the bowel lumen, leading to a decrease in intravascular volume, an increase in serum osmolarity, and a decrease in cardiac output. In patients with underlying bowel disease, additional injury is possible.13,14 Omnipaque (iohexol) is a nonionic water-soluble iodinated contrast medium that is available in concentrations of 140, 180, 240, 300, and 350 mg of iodine. It is poorly absorbed from the intact GI tract, with renal excretion of 0.1% to 0.5% of the administered dose. Isovue (iopamidol) also has been used in the evaluation of the pediatric GI tract, but currently only Omnipaque is officially approved for this purpose. It must be emphasized that the osmolality of both of these media is greater than that of blood and that no agent is safe in the tracheobronchial tree, and thus great care and close fluoroscopic monitoring is necessary in all patients in whom aspiration is a potential complication.15 Barium is the standard agent used in the evaluation of the colon. However, in cases of potential perforation, water-soluble agents are used and can be diluted to approximate the tonicity of serum. Higher osmolality contrast media are used rectally for therapeutic purposes in cases of uncomplicated meconium ileus after diagnosis with a low-osmolarity agent. Gastrografin (diatrizoate meglumine and diatrizoate sodium) was the original agent described for this purpose.16 However, this agent can be associated with large fluid shifts and systemic complications in severely ill infants.13 Full-strength iothalamate meglumine 30% also can be used successfully for this purpose. Close attention to water and electrolyte balance, along with surgical standby, are mandatory. Esophagram and Upper gastrointestinal Series The examination is begun in the lateral projection, with the child lying on his or her left side to maintain the ingested contrast agent within the fundus of the stomach. Images of the esophagus are obtained from the nasopharynx to the esophagogastric junction, with special attention paid to nasopharyngeal aspiration, tracheal aspiration, masses, fistulas, and esophageal peristalsis and distensibility. The child is then laid supine, and the esophagus is examined in the anteroposterior projection. When the evaluation of the esophagus is completed, the barium in the fundus will be directed into the duodenum by turning the child into the prone right anterior oblique position. Gastric emptying is assessed, along with distensibility of the antrum, pylorus, duodenal bulb, and descending duodenum. Once the contrast material has reached the junction of second and third portions of the duodenum, the child is quickly placed in the supine position for assessment of the duodenojejunal junction, which is visible through the air-filled antrum. The duodenojejunal junction should lie to the left of the spine, at approximately the same level as the duodenal bulb. Once this assessment is accomplished, the child is quickly turned again, this time for a lateral projection to document the posterior course of the ascending and descending limbs of the normally rotated retroperitoneal duodenum. Evaluation for reflux can be performed after this portion of the study, if desired, or this can be done through other means such as scintigraphy or esophageal probe. A final image documents gastric emptying (Fig. 85-1). Figure 85-1 Typical upper gastrointestinal series showing reflux in otherwise healthy infant. The primary role of sonography in the diagnosis of pyloric stenosis has become firmly established. Sonography is also extremely useful in the assessment of patients with clinically equivocal symptoms of appendicitis, although this setting punctuates its well-known operator dependence (e-Fig. 85-2), with published sensitivities ranging between 40% and 100%.17–19 Sonography also is extremely useful in the evaluation of mesenteric adenopathy (e-Fig. 85-3), in highly detailed assessment of the bowel wall (Fig. 85-4),20 in evaluation of small and large bowel intussusception (e-Fig. 85-5),21 and coupled with Doppler, in the effective estimation of disease activity in patients with Crohn disease.22 Figure 85-4 Ultrasound appearance of normal and abnormal loops of bowel. e-Figure 85-5 Small bowel intussusception. Although vascular structures are seen easily with contrast-enhanced CT, the direction and velocity of flow can be evaluated with Doppler sonography. Analysis of waveform pattern can identify hepatofugal flow in collateral vessels in patients with portal hypertension, along with vascular stenosis or thrombus. In patients with heterotaxy, abdominal sonography is helpful in assessing a splenic mass (located along the greater curvature of the stomach) and the associated vascular anomalies, such as interruption of the inferior vena cava, a preduodenal portal vein, and infradiaphragmatic total anomalous pulmonary venous connection23,24 (e-Fig. 85-6). e-Figure 85-6 Ultrasound findings in patients with heterotaxy. CT is a particularly useful modality in pediatric abdominal imaging. The introduction of scanners with multichannel technology and volumetric acquisition permits very rapid examinations with isotropic reconstructions in multiple planes, with decreasing need for sedation.25,26 These new capabilities require development of newer protocols to accommodate more complex and sophisticated diagnostic demands. The timing and rate of contrast administration, with the ability to scan during a specific phase of intravascular contrast distribution, demand particular attention to technical details and new approaches to image interpretation.27,28 The pediatric radiologist is further challenged by the need to balance image detail with radiation dose and implementation of the ALARA, or “as low as reasonably achievable” concept, with the increasing recognition of the potential risks of radiation exposure for pediatric patients.29,30 Improvements in equipment aimed at reducing radiation exposure include innovations such as improved collimators and iterative reconstructive algorithms. Although significant challenges persist, much progress has been made through educational and awareness-raising social marketing campaigns such as the Image Gently Campaign of The Alliance for Radiation Safety in Pediatric Imaging (www.imagegently.org).31 Unlike sonography, CT images are sequential, standardized, and much less operator dependent, and therefore CT is particularly useful in patients with complex disease affecting multiple organ systems, because it provides reliable monitoring of change in the extent of the disease during therapy and follow-up. CT also helps solve problems in patients with unusual multi-organ abnormalities. Evaluation of both intraabdominal and extraabdominal multiorgan pathology can be accomplished with great anatomic detail (e-Figs. 85-7 and 85-8) because evaluation of solid organ, hollow viscera, and peritoneal cavity pathology is rapidly accomplished with great anatomic detail and physiologic information. The relative lack of operator dependence and high sensitivity and specificity of CT in the imaging diagnosis of appendicitis has led to its increasing use when this diagnosis is clinically equivocal, with a documented reduction in negative appendectomies.32 However, this success has led to overuse in patients with abdominal pain; therefore physical examination, followed by ultrasound when the diagnosis is clinically uncertain, is recommended by most pediatric radiologists, with CT reserved for more difficult cases.17,18 MRI is receiving increasing attention as a viable substitute for CT scanning in many indications, such as inflammatory bowel disease.33 e-Figure 85-7 Pericardial hernia. e-Figure 85-8 A choledochal cyst with intrahepatic ductal dilatation and absence of the portal vein. CT protocols vary and undergo change with the ongoing introduction of new applications and advances in equipment capability; generalizable protocols applicable to pediatric patients can be downloaded at http://www.imagegently.org. However, some underlying principles underscore most successful pediatric examinations. Administration of intravenous contrast material is extremely important, particularly in pediatric patients in whom a paucity of intraabdominal fat decreases intrinsic intraabdominal contrast.27,34 CT angiography requires a rapid contrast bolus injection, which in pediatrics can be challenging because of the caliber of IV access. Lowering kVp is important in patients in whom high-contrast structures are of interest, such as those undergoing angiography or bone examinations; in neonates, the kVp can be decreased to as low as 80, with some adjustment of the milliamperes-second (mAs) to produce acceptable image quality.29 Precontrast images are seldom necessary and serve to increase the radiation exposure without adding diagnostic information. If necessary (e.g., to identify the presence of calcifications in an abdominal mass), the mAs of the precontrast scans can be decreased significantly and the scan should be limited to the appropriate specific area (e.g., scan only the mass, not the entire abdomen). The use of oral contrast material is usually important when outlining some types of intraperitoneal pathology, such as abscess or masses, but in other cases, its use is more controversial.35 Positive oral contrast material will mask mucosal enhancement; use of water-density contrast material may be more appropriate in such cases. Despite radiation concerns, CT remains an important life-saving modality in pediatric diagnosis. As with any other tool, it needs to be used judiciously, according to the principles of appropriateness, justification, optimization, and training.36 Use of an oral contrast agent is essential for enterography examinations. A number of choices are acceptable, although most regimens consist of a biphasic agent, that is, one that gives the bowel lumen a long T2 and T1 relaxation time. Agents include VoLumen (E-Z-Em, New York, NY), mannitol, polyethylene glycol l, and locust bean gum (a type of galactomannan) solutions. Little difference is seen in efficacy of these choices, although patient tolerance may vary.37 Most important is rapid consumption of a large volume of the contrast agent; 25 mL per kilogram of body weight over an hour is adequate. Placing the patient in the right decubitus position for the final 15 minutes before the start of imaging aids in emptying of the stomach. Equipment specifications are important because of children’s smaller sizes, with the consequent need for improved signal to noise and faster acquisition times to decrease the need for and length of sedation. Although the literature to date is still sparse on pediatric abdominal imaging at 3 T,38,39 increasing experience suggests that most children will benefit from the higher signal. Phased array surface coils are now standard, typically with eight to 32 channels. In the following situations, 1.5 T often provides improved image quality compared with 3 T: when the patient is very large, when ascites is present, when enterography examinations are being performed (1.5 T results in fewer banding artifacts in steady-state imaging), and during hepatic iron quantification. Hepatic tumors are well evaluated by MRI relative to CT,40 with the goal of imaging being tumor characterization, staging, and assessment of resectability. For lesion characterization, determination of the T2-weighted signal and enhancement characteristics is essential.41 Staging and resectability of tumors (such as hepatoblastomas) require delineation of anatomic boundaries, lymph node involvement, vascular invasion, and delineation of the biliary tree, according to accepted staging systems such as PRETEXT (PRETreatment tumor EXTension) outlined by the International Childhood Liver Tumor Strategy Group.42,43 Biliary and pancreatic diseases also are well assessed by MRI.44,45 Common indications include cholelithiasis, pancreatitis, sclerosing cholangitis,46,47 ductal plate malformations and choledochal cysts,48–50 and biliary complications of liver transplantation. In the case of liver transplantation, assessment of vascular complications often is essential. Diffuse liver disease, such as fibrosis, steatosis,51–53 and iron deposition, can be quantified by MRI. Fibrosis has been quantitatively assessed by elastography,54 as well as qualitatively by T2-weighted imaging and delayed contrast enhancement.55 Although steatosis can be assessed by spectroscopic methods,56 more commonly steatosis, as well as iron deposition, are assessed by multi-echo gradient echo imaging.53,57 MR enterography is most commonly performed for evaluation of inflammatory bowel disease.58–60
Imaging Techniques
Plain Films and Fluoroscopy
Indications and Protocols
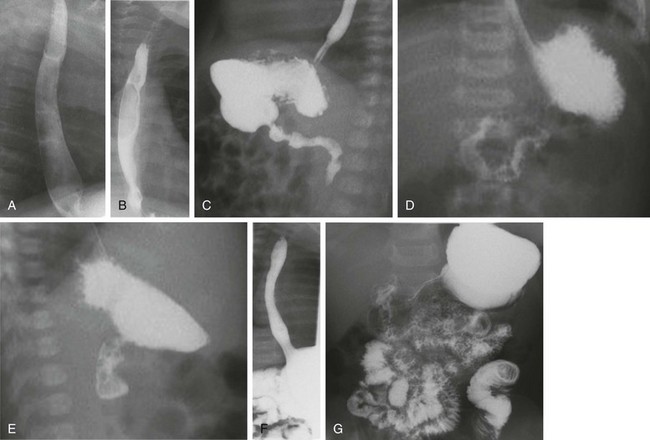
A and B, Lateral and anteroposterior views of the esophagus during drinking show full distensibility without intrinsic or extrinsic mass lesions. C, Oblique imaging (right anterior oblique) directs ingested contrast material to the gastric outlet and shows prompt emptying and a normal pylorus and first and second portions of the duodenum. D, An anteroposterior image immediately following C shows progress of contrast material to the normally located gastroduodenal junction at the ligament of Treitz. E, A subsequent lateral view of the duodenum shows the posterior retroperitoneal location of parallel ascending and descending limbs. F, After further drinking and gastric filling, an episode of reflux to the cervical esophagus is documented. Note the wide open gastroesophageal junction, a typical appearance during reflux. G, An image recorded at the completion of the study shows good progress of contrast material through the small bowel.
Sonography
Indications and Protocols
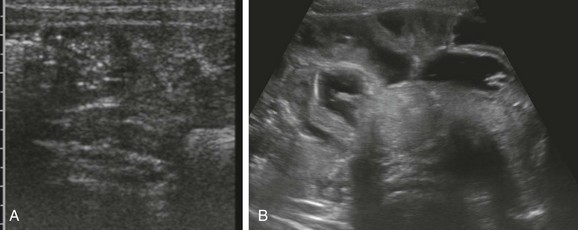
A, A transverse image through the right lower quadrant demonstrates normal, collapsed loops of small bowel. B, A transverse image through the mid abdomen in a 14-month-old boy with vomiting and diarrhea consistent with gastroenteritis reveals multiple distended, fluid-filled loops of bowel.
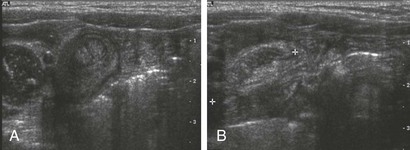
Transverse (A) and longitudinal (B) images through incidental small bowel intussusception in a 7-month-old infant with gastroenteritis. The length between calipers in B was 2 cm. A follow-up study several hours later showed resolution of the intussusception, and the child remained asymptomatic.
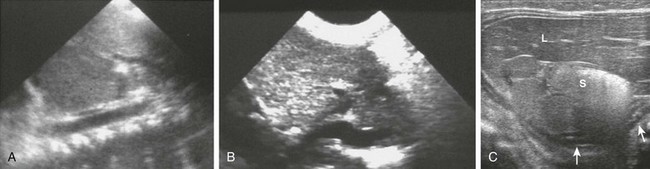
A, A preduodenal portal vein. A longitudinal image through the upper abdomen in a child with polysplenia shows the course of the superior mesenteric vein into the liver anterior to the gas-filled duodenal bulb. B, Infradiaphragmatic total anomalous pulmonary venous connection. A longitudinal image through the upper abdomen in an infant with asplenia shows the anomalous vessel entering the abdomen from the chest at the esophageal hiatus. C, Multiple splenules in 1-day-old girl with abnormal results of a prenatal ultrasound. Transverse ultrasound of the right upper quadrant reveals multiple splenules between the stomach (S) and the ipsilateral adrenal gland (arrows). Azygous continuation of the inferior vena cava was documented elsewhere. She did not have congenital heart disease. L, Lateral extension of liver, the bulk of which was in the left upper quadrant.
Computed Tomography
Indications and Protocols
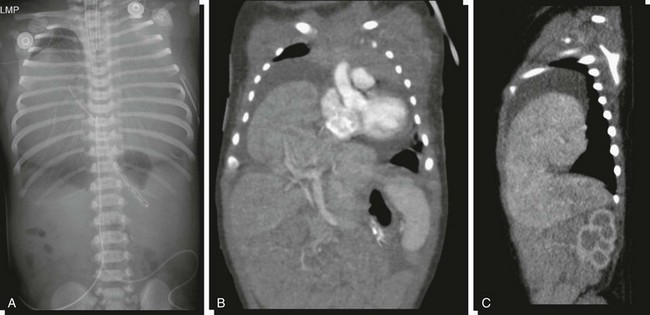
A, An abdominopelvic radiograph of a newborn infant with a prenatal diagnosis of hydrothorax. The radiograph suggests absence of most of the liver shadow from the right upper quadrant. The posterior costophrenic sulci are well outlined, indicating that chest density is not related to bilateral hydrothorax. Coronal (B) and sagittal (C) reformats of contrasted computed tomography scans outline herniation of the liver into the pericardial cavity with displacement of the heart and associated pericardial effusion.
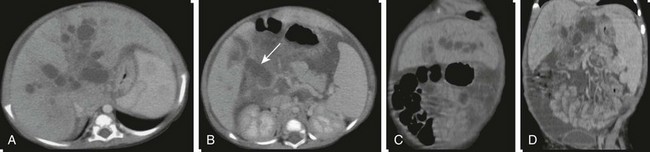
A and B, Axial contrasted computed tomography images through the liver and porta hepatis outline intrahepatic cystic dilatation of the biliary tree (Caroli disease) and cystic dilatation of the common bile duct at the porta hepatis (arrow). C and D, Coronal reconstructions better define the cystic intrahepatic ductal dilatation, absence of the portal vein, and splenomegaly.
Magnetic Resonance Imaging
Patient Preparation and Equipment Requirements
Indications and Protocols
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Imaging Techniques