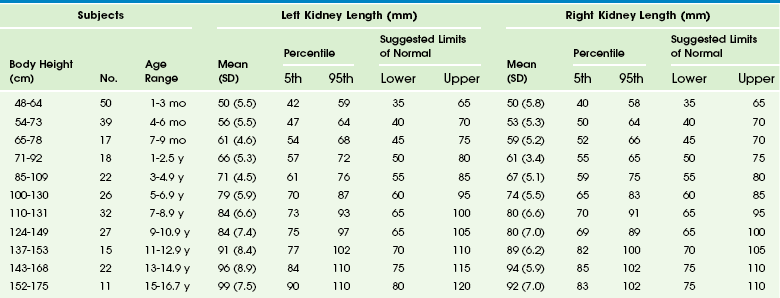
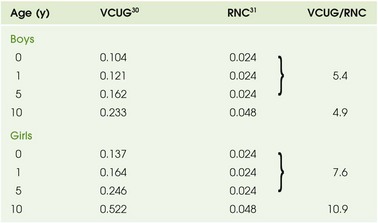
Chapter 112 Imaging begins with a frontal radiograph of the abdomen to identify any calcifications or masses. After this preliminary radiograph is obtained, low osmolar contrast media with a high iodine content is administered intravenously at a dose of 2 mL/kg (maximum, 150 mL) to obtain adequate iodine concentration in the renal tubules and collecting system. The filming sequence is tailored to the individual examination. An initial frontal radiograph within 1 to 2 minutes of injection images the nephrographic phase. Assessment of this radiograph determines subsequent filming. Upon routine examination, a radiograph at approximately 5 to 10 minutes allows visualization of the kidneys and their collecting systems, including the bladder (e-Fig. 112-1). In the prone position, the higher specific gravity of the contrast material allows better visualization of the anteriorly positioned renal pelves and proximal ureters. e-Figure 112-1 A normal intravenous urography image. Antegrade voiding cystourethrography (VCUG) is the traditional examination of choice for detailed anatomic evaluation of the bladder, study of the anatomy of the male urethra, and identification of vesicoureteral reflux (VUR). The bladder is filled by gravity pressure, using dilute sterile contrast media with an iodine concentration of 80 to 100 mg/mL. The predicted bladder capacity (in milliliters) for children younger than 1 year is the child’s weight in kilograms multiplied by 7. In children older than 1 year, the predicted capacity is the child’s age in years plus 2, multiplied by 30.1,2 An early bladder filling image is obtained to evaluate for ureteroceles or masses. Images with a full urinary bladder are obtained in the lateral oblique projections to look for VUR. Voiding films are useful to evaluate the bladder and urethra (particularly the male urethra) and for the diagnosis of VUR, which may occur only during voiding. After voiding, an image of the bladder documents any postvoid residual, and an image of the kidneys documents any reflux that occurred during the examination. Neonates should undergo at least two filling and voiding cycles to increase the chance of detecting VUR.3–6 Pulsed fluoroscopy, last image hold recording, and videotaping are important imaging strategies for reducing radiation exposure.7–10 Contrast-Enhanced Voiding Ultrasonography The intravesical instillation of ultrasound contrast agents in the urinary bladder allows the sonographic evaluation of VUR without the use of ionizing radiation.11–14 These microbubble contrast agents are composed of an outer shell of lipid, protein, or polymer that encases a gas, most commonly a perfluorocarbon.15 The gas is highly reflective on ultrasound imaging (Fig. 112-2) and can be detected even when administered in very small volumes. The ultrasound transducer is positioned intermittently over the bladder, ureters, and kidneys while the bladder is filled. On grayscale imaging, the microbubbles appear echogenic. Refluxed contrast material is easily detected in the ureters and kidneys (Fig. 112-3). Although this technique does not avoid catheterization, it does eliminate radiation exposure. Results indicate that the sensitivity for VUR detection is comparable with that of standard techniques. A reflux grading system for contrast-enhanced voiding ultrasonography has been developed and is similar to the international grading system for VCUG.12 Urethral visualization is possible with contrast-enhanced voiding ultrasonography but remains challenging. Figure 112-2 Contrast-enhanced cystosonography. Figure 112-3 Contrast-enhanced cystosonography. Ultrasonography is an ideal method for examining the kidneys and bladder in infants and children because of their small physical habitus and lack of abdominal fat and because ultrasonography does not utilize ionizing radiation. Variable transducer frequencies and transducer design (e.g., sector, phased, curvilinear, and linear array) allow for individualized approaches. Doppler ultrasound is valuable for the detection of blood flow, to confirm arterial perfusion, or to exclude venous thrombosis. Measurable blood flow parameters from spectral Doppler analysis include peak systolic velocity, end-diastolic velocity, and acceleration times. The normal renal artery has a prompt systolic upstroke with an acceleration time of 70 msec or less and a visible early systolic peak (e-Fig. 112-4). The normal resistive index depends on the patient’s age; it may be as high as 0.9 in a preterm infant and falls to around the adult value of 0.7 in the first few months of life.16–19 e-Figure 112-4 A normal renal Doppler waveform. In young children, it is advisable to initiate the urogenital ultrasound examination with an examination of the bladder. The full bladder of an infant usually empties when the transducer is placed in the suprapubic region. Kidneys are imaged in the longitudinal and transverse planes. The kidneys are ovoid solid organs with fine, medium-level echoes arising from the cortex, a well-delineated corticomedullary junction with brightly echoic arcuate arteries, and pyramid-shaped, relatively large medullary rays that are hypoechoic. Cortical echogenicity in neonates and young infants is higher and the medullary pyramids are more hypoechoic than in older children (Fig. 112-5). The cortical echogenicity is increased compared with the liver and spleen in preterm infants, isoechoic in neonates and young infants, and diminishes progressively in older children. The transition from the infant renal echo pattern to that of the child typically occurs between 6 and 9 months (Fig. 112-6). Normal pediatric sonographic measurements of right and left kidney length, based on height and age, are provided in Table 112-1.20–25 Table 112-1 Normal Sonographic Renal Lengths in Children Based on Height and Age From Konus OL, Ozdemir A, Akkaya A, et al. Normal liver, spleen, and kidney dimensions in neonates, infants, and children: evaluation with sonography. AJR. 1998;171:1693-1698. Figure 112-5 A normal newborn kidney. Nuclear cystography is performed for the assessment of VUR and is an alternative to fluoroscopic VCUG. The examination is performed by direct instillation of radiotracer (technetium-99m–sulfur colloid) and sterile saline solution into the bladder after sterile catheterization26 or indirectly after nuclear renography with planar images obtained during voiding.27 Dynamic imaging of the bladder and kidney regions is acquired with use of a posterior gamma camera throughout the filling and voiding cycle. The data can be grouped (in 10- or 60-second intervals) and viewed dynamically. VUR is documented when tracer is shown to ascend into a tubular structure corresponding to the ureter or when the renal collecting system is visualized. The dose of tracer is dependent on bladder volume: 300 mCi for bladder volumes up to 300 mL, and 600 mCi for larger bladder volumes. A cyclic cystogram is recommended for children younger than 2 years, for children with previously documented VUR or a high suspicion for VUR, and for children who void well before the expected bladder capacity is reached. The procedure is identical to the standard cystogram; however, the catheter is left in the bladder after the first voiding cycle and is used to refill the bladder for a repeat void. As with VCUG, cyclic studies increase the diagnostic yield (e-Fig. 112-7), identifying an additional 10% to 15% of children with VUR compared with noncyclic voiding studies.28 e-Figure 112-7 Vesicoureteral reflux. Nuclear cystography offers three main advantages over fluoroscopic VCUG: increased detection of VUR (up to an additional 20%) (Fig. 112-8), frequent detection of a higher grade of VUR,29,30 and reduced radiation dose (tenfold) (Table 112-2). Disadvantages of nuclear cystography include a lack of detailed anatomic visualization of the urethra and collecting systems and limited identification of bladder abnormalities (such as periureteric diverticula); in addition, the classification of VUR with nuclear cystography is less refined than that with VCUG. The nuclear cystography VUR grades of low, intermediate, and high roughly correspond to the fluoroscopic grades of 1 (low), 2 or 3 (intermediate), and 4 or 5 (high).26 Table 112-2 Effective Radiation Doses (mSv) for Boys and Girls RNC, Radionuclide cystogram; VCUG, voiding cystourethrogram. Figure 112-8 Vesicoureteral reflux. Diuretic renography is used to distinguish obstructive from nonobstructive hydronephrosis.31 It attempts to quantify urinary obstruction based on the relative function of the hydronephrotic kidney compared with the normal kidney and the rate of urinary excretion of radiotracer (technetium-99m mertiatide or technetium-99m diethylene triamine pentaacetic acid) from the renal pelvis (and, in the presence of hydroureter, from the ureter) after a diuretic challenge (1 mg/kg IV furosemide). The graphic presentation of renal excretion using a time versus intensity curve is termed a renogram (e-Fig. 112-9); normal, equivocal, and obstructed patterns of excretion after a diuretic challenge, termed washout, have been described31 (Fig. 112-10). Additionally, the time required for half the tracer in the collecting system to pass across the ureteropelvic junction after the administration of furosemide, termed diuretic T½, is stratified to indicate a normal (0 to 10 minutes), equivocal (10 to 20 minutes), or obstructed (>20 minutes) pattern.32,33 These values are useful in distinguishing obstructive from nonobstructive hydronephrosis in older children and adults. However, application of these guidelines can lead to the misdiagnosis of obstruction in a large number of young infants with hydronephrosis demonstrated on routine prenatal sonography (e-Fig. 112-11).34 The high capacitance of the dilated renal pelvis and relatively low renal urine output in young infants limit the accuracy of this test in the setting of hydronephrosis in children younger than 2 years.33 Increasing hydronephrosis, decreasing split renal function of the hydronephrotic kidney, and a worsening washout curve all suggest the possibility of significant obstruction (Fig. 112-12). Figure 112-10 Diuretic renogram patterns. Figure 112-12 Hydronephrosis. e-Figure 112-9 A time versus activity graph shows a steady accumulation of tracer in the renal region of interest until washout occurs, starting 3 minutes after injection of a diuretic agent (arrow).
Imaging Techniques
Radiographic Procedures
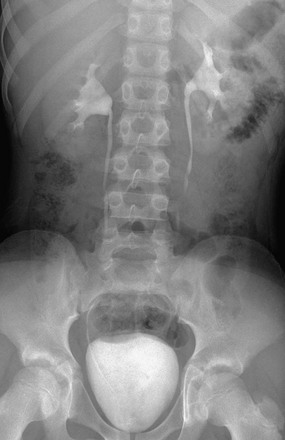
A 5-minute image shows opacification of the upper collecting system with normal thin, delicate calyces, normal nondilated ureters, and filling of the bladder.
Voiding Cystourethrography
Ultrasound
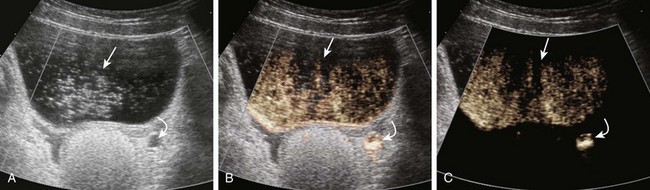
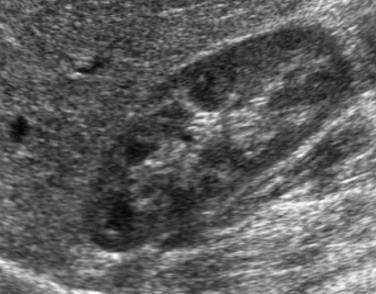
Transverse ultrasound images after instillation of an ultrasound contrast agent into the urinary bladder. A, On gray-scale imaging, the contrast agent appears as hyperechoic material in the bladder (straight arrow) surrounded by anechoic urine. Reflux into the distal left ureter (curved arrow) is present but somewhat difficult to appreciate. B, Color overlay technology shows the contrast agent to better advantage as bright orange material in the bladder (straight arrow) and distal ureter (curved arrow). C, Subtraction technology further accentuates the presence of contrast material by eliminating non–contrast-enhanced background tissue. Contrast material in the bladder (straight arrow) and ureter (curved arrow) are readily apparent. (Images courtesy Dr. Kassa Darge.)
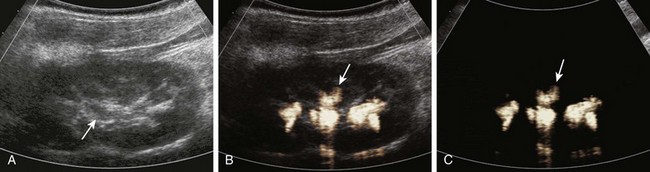
Longitudinal ultrasound images of the kidney, obtained during contrast-enhanced voiding ultrasonography, demonstrate reflux into the renal collecting system (arrows) on gray-scale (A), color-overlay (B), and subtraction (C) images. (Images courtesy Dr. Kassa Darge.)
Renal Ultrasonography
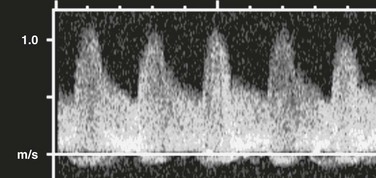
A prompt systolic upstroke and normal forward flow through diastole are present.
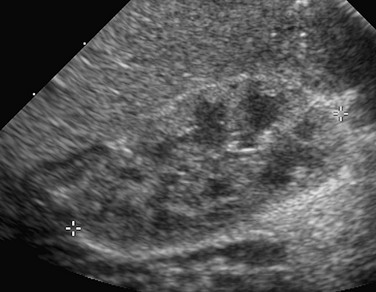
A longitudinal sonogram shows that the right renal cortex is slightly more echogenic than the adjacent liver. The hypoechoic medullary pyramids are quite distinct.
Nuclear Medicine

A cyclic nuclear cystogram demonstrates a normal first cycle, with intermediate-grade reflux present on the second cycle only (arrow).
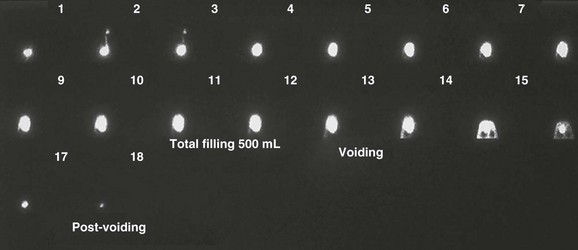
Early in the study, this nuclear cystogram shows intermediate-grade vesicoureteral reflux that later fully drains and does not recur through voiding. Continuous acquisition of the nuclear cystogram allowed the demonstration of this transient reflux, which likely would have been missed with fluoroscopic voiding cystourethrography.
Diuretic Renography
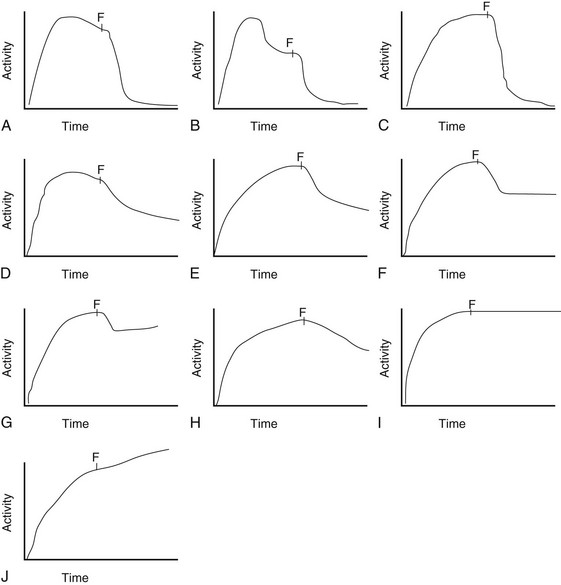
Patterns A, B, and C are typically indicative of nonobstructed systems. Patterns D and E are equivocal in older children but usually indicate no obstruction in neonates and young infants with hydronephrosis. Patterns F and G often indicate flow-related obstruction. Patterns H, I, and J typically indicate obstruction in older children but are seen frequently in neonates and infants with nonobstructive hydronephrosis. F, Furosemide injection.
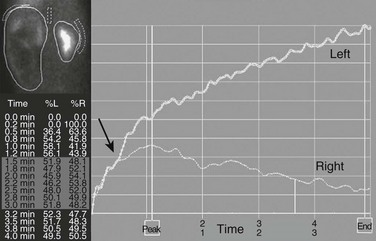
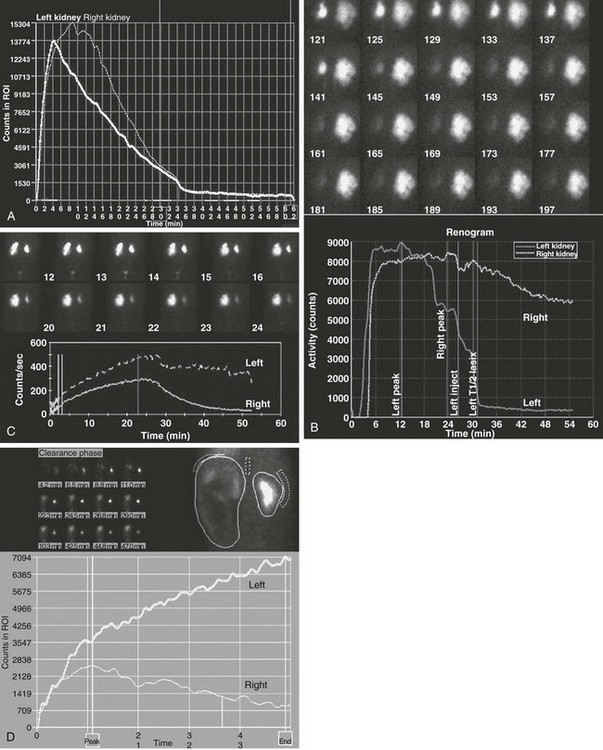
A markedly enlarged left kidney with central photopenic regions consistent with marked hydronephrosis. The renogram shows tracer accumulation and retention throughout, with no discernible washout after administration of a diuretic. Note that the renogram tracings of the two kidneys are superimposed during the first few minutes after injection of the tracer (arrow), which indicates nearly equal split renal function, as shown on the function table between 1.5 and 3 minutes.
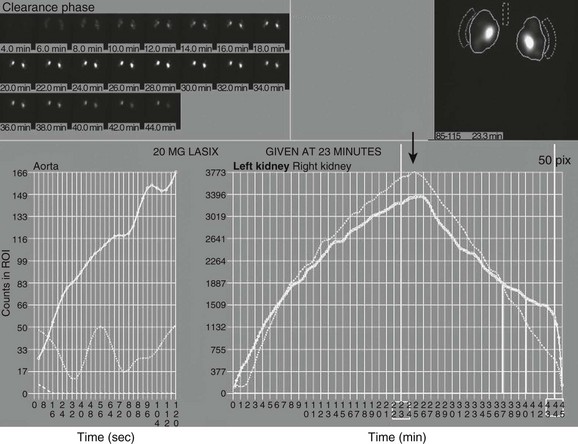
Note the superolateral background region of interest adjacent to both kidneys. Also note mild urinary stasis and hydronephrosis, with no spontaneous excretion until after administration of a diuretic agent. The diuretic T½ is approximately 10 minutes, which is considered normal in the presence of mild hydronephrosis.
Imaging Techniques