Fig. 3.1
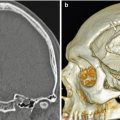
Cortical bone reconstruction. 3D CT image shows cortical bone graft positioned along the expected site of the nasal dorsum (arrow) and absence of the native nasal bones and frontal processes of the maxilla
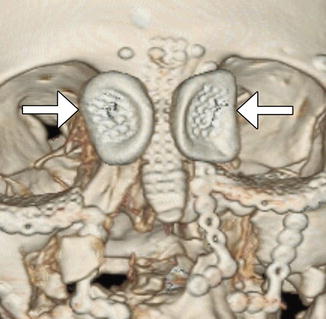
Fig. 3.2
Nasal fracture reconstruction with low-profile mesh. 3D CT image shows a mesh positioned along the nasal dorsum secured via low-profile screws. There are also bilateral molded polyvinyl siloxane plates (arrows) fit over the nasal soft tissue in the medial canthal area and secured via transcutaneous wires
3.2 Septoplasty
3.2.1 Discussion
Septoplasty is performed to treat a deviated nasal septum and can be performed in conjunction with rhinoplasty (septorhinoplasty). Classic septoplasty consists of creating a mucoperichondrial flap in order to remove the offending portion of the nasal septum via sharp dissection (Fig. 3.3). Silastic sheets or stents are often inserted along both sides of the nasal septum to prevent the formation of adhesions (Fig. 3.4). These are later removed once the surgical site heals. The postoperative imaging appearance often consists of a straightened and thinned nasal septum with widened nasal passages, which can be subtle. Complications are uncommon and include hemorrhage, cerebrospinal fluid leak, infection, septal hematoma or abscess, overcorrected septum, septal perforation (Fig. 3.5), adhesions, and sensory disturbances.
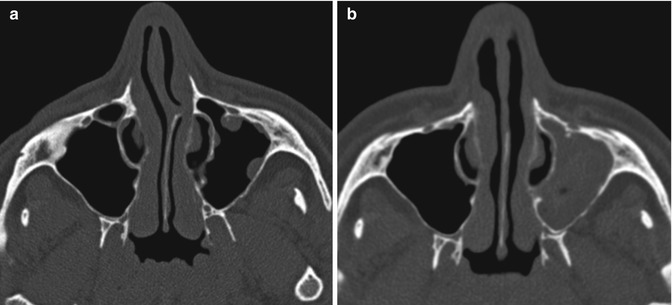
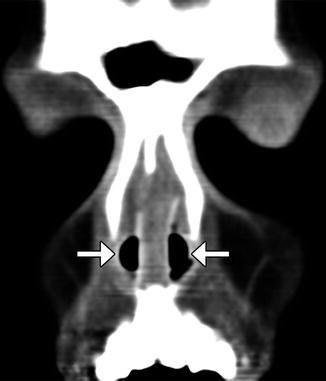
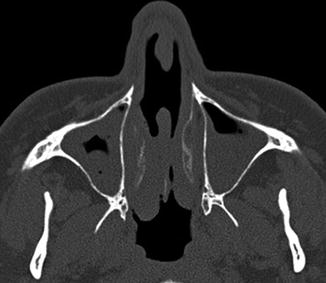

Fig. 3.3
Septoplasty. The patient has a history of a deviated nasal septum with spur causing nasal obstruction. Preoperative axial CT image (a) shows leftward deviation of the nasal septum with a spur. Axial CT image obtained 1 year after surgery (b) shows interval removal of the spur and straightening of the nasal septum. There is also increased opacification of the left maxillary sinus

Fig. 3.4
Nasal stents. The patient has a history of nasal septal deviation and adhesions treated via septoplasty and lysis of adhesions. Coronal CT image shows a straight, midline septum flanked by bilateral nasal stents (arrows)

Fig. 3.5
Septoplasty perforation. Axial CT image shows a defect in the anterior nasal septum. The septum is otherwise straight. However, there is acute sinusitis
3.3 Nasal Septal Button Prosthesis
3.3.1 Discussion
Nasal septal perforation may present with various symptoms: epistaxis, crusting, secondary infection, whistling, and nasal obstruction. Perforation can be treated by conservative pharmacological treatment or by surgical closure. Alternatively, a nasal septal button, often composed of silicone, can be inserted transnasally to span the perforation (Fig. 3.6).
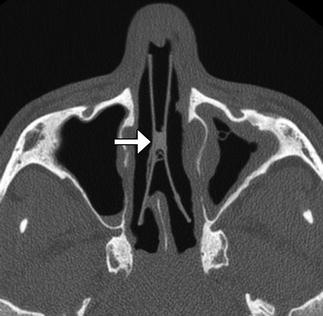

Fig. 3.6
Nasal septal button prosthesis. Axial CT image shows the two discs connected to one another across the nasal septal defect (arrow)
3.4 Inferior Turbinate Outfracture and Reduction
3.4.1 Discussion
Inferior turbinate outfracture and reduction are treatment options for nasal obstruction related to inferior turbinate hypertrophy. Outfracture consists of laterally displacing the inferior turbinates, while radio-frequency treatment coagulates the inferior turbinate submucosa. This results in a widened nasal airway passage. The procedures are often performed in conjunction with septoplasty. Lateral displacement of the anterior, middle, and posterior portions is usually apparent on postoperative sinus CT (Fig. 3.7). Alternatively, turbinate reduction surgery typically results in a truncated appearance of the inferior turbinates and enlargement of the nasal passages (Fig. 3.8).
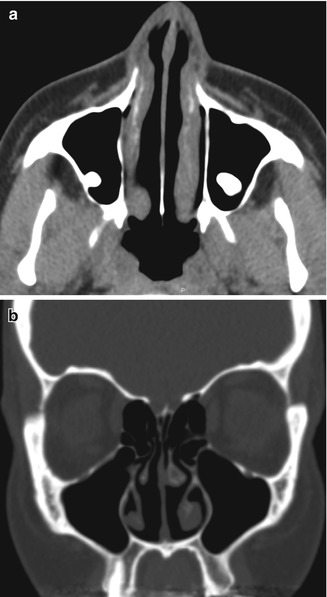
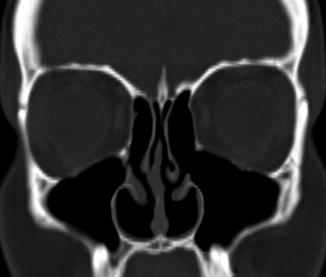

Fig. 3.7
Inferior turbinate outfracture. Axial (a) and coronal (b) CT images show lateral deviation of the bilateral inferior turbinates with reduced mucosa and evidence of septoplasty, which result in a widened nasal passage

Fig 3.8
Inferior turbinate reduction. Coronal CT image shows truncation of the inferior portions of the bilateral inferior turbinates. Findings related to endoscopic sinus surgery are also apparent
3.5 Nasal Packing Material
3.5.1 Discussion
Nasal packs are routinely used in sinonasal surgery in order to apply pressure, fill preformed spaces, create moist environments to facilitate physiological processes, function as a barrier, and induce physiological hemostatic and reparative processes. Nasal packings, including Merocel and MeroGel packs, and alginate strips have a tendency to imbibe blood products in the early perioperative period, which is reflected in the appearance on imaging (Fig. 3.9). Bismuth and iodoform paraffin paste using some packing material displays high CT attenuation that results in severe image degradation. Aqueous Betadine gauze also displays high attenuation on CT. Myospherulosis, a foreign body-type granulomatous reaction to lipid-containing material, has a characteristic fat-attenuation appearance on CT.
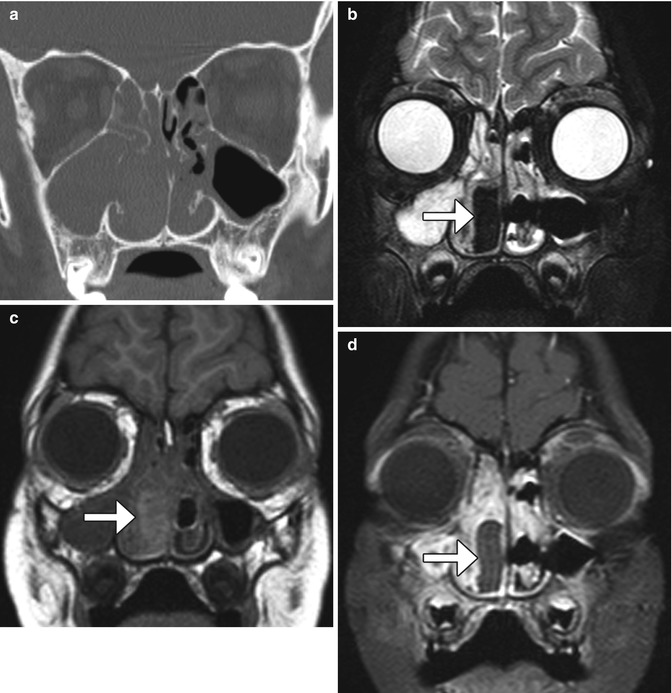

Fig. 3.9
Nasal packing. Coronal CT image (a) shows opacification of the right nasal cavity and paranasal sinuses. Coronal T1-weighted MRI (b) shows that the nasal packing material is very hypointense (arrow). The T1-weighted (c) and fat-suppressed post-contrast T1-weighted (d) MR images show that the nasal packing (arrows) is mildly T1 hyperintense and does not enhance, unlike the surrounding mucosa
3.6 Rhinectomy
3.6.1 Discussion
Rhinectomy is an oncological procedure that involves resecting part or all of the external nose when involved by high-risk nasal malignancies. Prosthetic rehabilitation can be an alternative to flap reconstruction, particularly in those patients unsuitable for major reconstruction. Customized prostheses can be created via computer-aided design based on preoperative virtual laser scanning or CT of the affected site adapted to the postoperative laser-scanned surface or CT (Fig. 3.10).
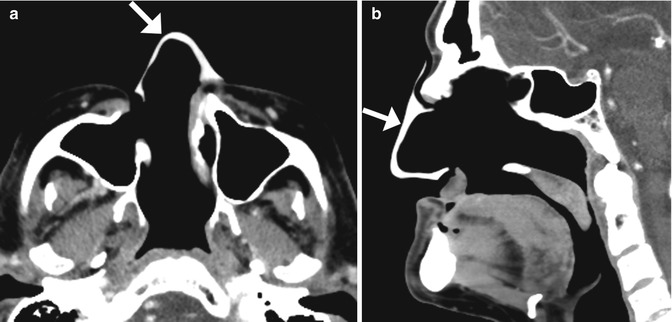

Fig. 3.10
Total rhinectomy with nose prosthesis. The patient had a history of nasal squamous cell carcinoma. Axial (a) and sagittal (b) CT images show a total rhinectomy defect that was reconstructed using a custom-made silicone prosthesis (arrows)
3.7 Sinus Lift Procedure
3.7.1 Discussion
The sinus lift or augmentation procedure is performed to build up a deficient maxillary alveolus for subsequent osseointegrated dental implant insertion. The procedure involves accessing the maxillary sinus and elevating the mucous membrane at the inferior aspect of the maxillary to create a space in the floor of the sinus where bone graft material is inserted, typically with a dome-shaped configuration on coronal images (Fig. 3.11). The bone graft initially has a porous appearance but consolidates with a more uniformly hyperattenuating appearance on CT as osseointegration and osteogenesis ensure after 6–8 months. Disruption of the sinus mucosa may result in sinusitis, graft infection, or formation of oroantral fistula, which is best depicted on coronal plane CT images (Fig. 3.12). Furthermore, graft material scattered in the sinus may also indicate surgical failure.
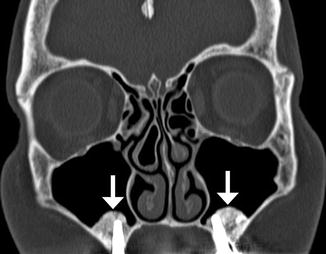
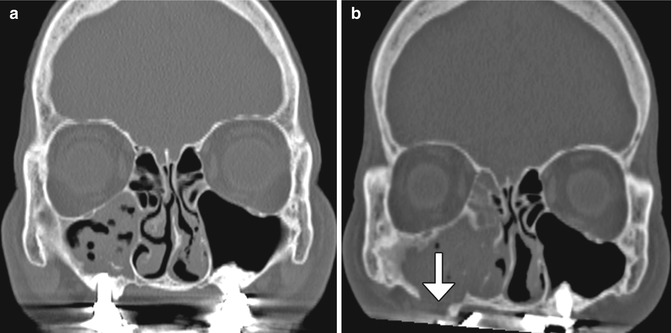

Fig. 3.11
Sinus lift procedure. Coronal CT image shows bone graft material (arrows) within the inferior maxillary sinuses adjacent to the alveolar ridge. There are bilateral osseointegrated implants

Fig. 3.12
Sinusitis and oroantral fistula after sinus lift procedure. Coronal CT image (a) shows bilateral sinus lift procedures with osseointegrated implants and opacification of the right maxillary sinus. Follow-up CT image (b) shows a defect in the maxillary sinus floor (arrow) and persistent right-sided sinus opacification
3.8 Caldwell-Luc Procedure
3.8.1 Discussion
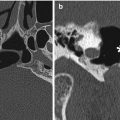
The Caldwell-Luc procedure was described by Caldwell in 1893, Spicer in 1894, and Luc in 1897 for the treatment of maxillary sinusitis. The technique originally consisted of creating a defect in the inferior aspect of the anterior maxillary wall via a canine fossa approach and removing diseased mucosa from the maxillary sinus, combined with inferior or middle meatus antrostomy, in order to facilitate gravitational intranasal counterdrainage and antral lavage (Fig. 3.13).
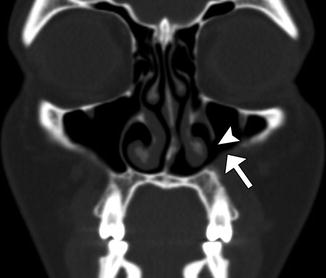

Fig. 3.13
Caldwell-Luc surgery. Coronal CT image shows a defect in the left anterior maxillary sinus wall (arrowhead) and nasoantral wall (arrow)
Historically, the Caldwell-Luc procedure was the primary sinus surgery until it was largely supplanted by functional endoscopic sinus surgery. The Caldwell-Luc procedure has fallen out of favor since it interrupts the ciliary clearance mechanism of the maxillary sinus mucosa. As a result, the procedure often exacerbates the conditions that it is intended to treat. Indeed, on postoperative imaging, inflammatory sinus disease, sinus collapse, and sinus wall sclerosis (osteitis) are found in over 80%, over 90%, and up to 100% of cases, respectively (Fig. 3.14). Currently, modification of the Caldwell-Luc procedure is mainly reserved as an approach for resection of selected maxillary sinus tumors and antrochoanal polyps.
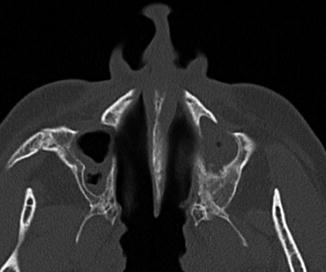

Fig. 3.14
Chronic recurrent sinusitis after bilateral Caldwell-Luc surgery. Axial CT shows the bilateral postoperative changes with mucosal thickening and hyperostosis of the remaining maxillary sinus walls
3.9 External Ethmoidectomy
3.9.1 Discussion
In the past, before the advent of functional endoscopic surgery techniques, resection of the ethmoid labyrinth was commonly performed for the treatment of sinusitis via a transorbital approach. The lamina can also be removed endoscopically for tumors such as inverted papillomas. The surgery involves resection of the ipsilateral lamina papyracea through which the paranasal sinuses can be visualized and accessed. The resulting defect in the lamina papyracea can be substantial (Fig. 3.15). Although external ethmoidectomy has been largely supplanted by FESS for treating rhinosinusitis, the approach may still be implemented for resecting certain sinonasal tumors.
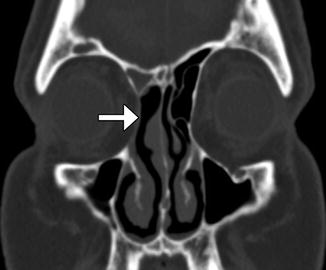

Fig. 3.15
External ethmoidectomy. Coronal CT image shows a defect in the right lamina papyracea (arrow), through which the right ethmoid air cells were resected. There is also right frontal blockage, which can be a complication of this approach
3.10 Functional Endoscopic Sinus Surgery
3.10.1 Discussion
Functional endoscopic sinus surgery (FESS) is used to treat chronic sinusitis and is occasionally performed as part of tumor resection. The objective of FESS is to relieve obstruction of mucus drainage while preserving the mucociliary clearance mechanism. The procedure consists of resecting various portions of the paranasal sinuses using an intranasal endoscope depending on the extent of disease and whether the anterior or posterior drainage routes are predominantly affected. The resulting changes are not necessarily symmetric from right to left or reproducible from one patient to another. Nevertheless, certain fundamental strategies are generally implemented, which are based on the major mucosal drainage pathways. CT with multiplanar reformatted images is the first-line modality for evaluating the paranasal sinuses and surrounding structures following FESS.
Turbinoplasty or partial anterior middle turbinectomy is sometimes performed in order to increase the exposure of the paranasal sinuses. The middle turbinate can be completely resected if it is responsible for obstructing the middle meatus (Fig. 3.16).


Fig. 3.16
Middle turbinectomy. Coronal CT image shows resection of the left middle turbinate, leaving behind a portion of the left vertical lamella (arrow). There is a concha bullosa on right side
Uncinectomy is essentially performed during all types of FESS when the ostiomeatal complex is affected by rhinosinusitis, along with resection of variable amounts of the anterior ethmoid air cells. Since the anterior ethmoid air cells normally comprise two-thirds to three-quarters of the ethmoid air cells, the resection cavity can extend rather far posteriorly. Typically, anterior ethmoidectomies and uncinectomies are performed together in order to optimally decompress the ostiomeatal complex and access the maxillary sinuses (Fig. 3.17).


Fig. 3.17
Typical pattern of ostiomeatal unit FESS. Coronal CT image shows the absence of the bilateral anterior ethmoid air cells and uncinate processes, resulting in widely patent anterior drainage pathways and clear sinuses
Disease of the posterior drainage system can be treated via ethmoidectomy alone or in combination with sphenoidotomy, which consists of enlarging the sphenoid sinus ostium (Figs. 3.18 and 3.19). This is often performed in conjunction with decompression of the ostiomeatal complex.
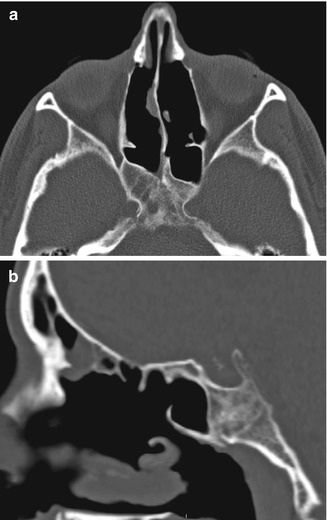
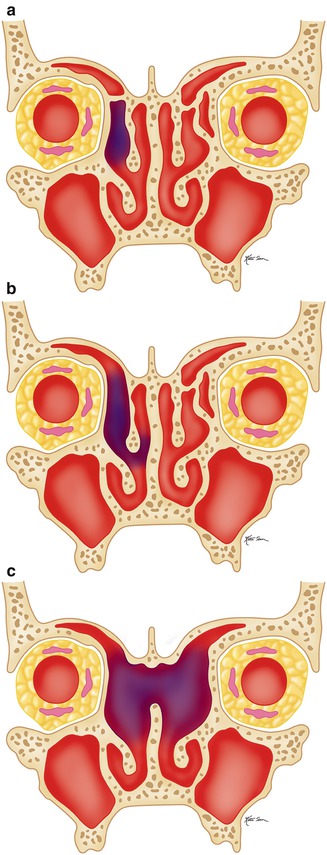

Fig. 3.18
Posterior drainage pathway FESS. Axial (a) and sagittal (b) CT images show bilateral enlarged sphenoid ostia (arrows). Bilateral total ethmoidectomies and middle turbinectomies were also performed

Fig. 3.19
Illustration of the types of frontal sinusotomy. Draf type I (a), Draf type II (b), and Draf type III (c)
Disease that affects the frontoethmoid drainage pathway can be addressed via frontal recess sinusotomy. Frontal recess sinusotomy approaches have been traditionally classified as Draf type I through III based on the extent of agger nasi and frontal air cells resected (Figs. 3.19, 3.20, 3.21, and 3.22). The Draf type III (modified Lothrop) procedure is the most radical form of frontal sinusotomy and involves resection of the upper internasal septum in addition to the frontal air cells.
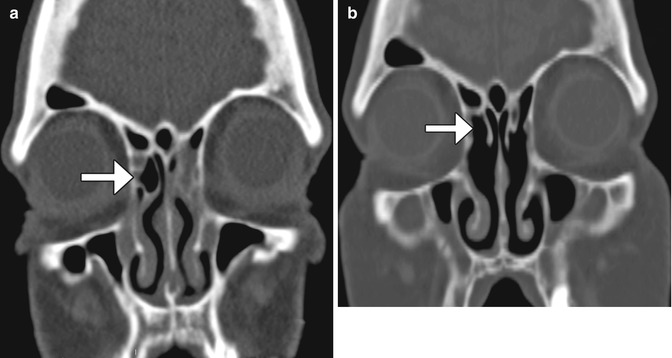
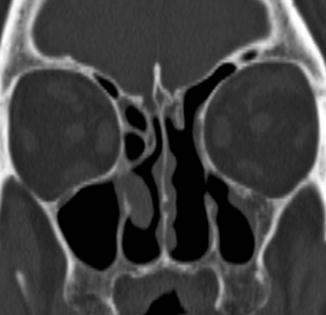
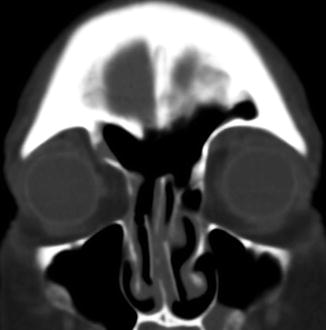

Fig. 3.20
Draf type I frontal sinusotomy. Preoperative coronal CT image (a) shows a partially opacified right agger nasi cell (arrow). Postoperative coronal CT image (b) shows a defect in the inferior aspect of the right agger nasi cell (arrow)

Fig. 3.21
Draf type II frontal sinusotomy. Coronal CT image shows a complete absence of the air cells in the left frontoethmoidal sinus drainage pathway, as compared to the intact contralateral side. Turbinate resection was also performed

Fig. 3.22
Draf type III frontal sinusotomy (modified Lothrop). Coronal CT image shows contiguous bilateral enlargement of the frontal sinus floors and resection of the interfrontal sinus septum and superior nasal septum. Septoplasty was also performed with Silastic plates in position
Occasionally, a defect is created in the medial maxillary sinus wall (antrostomy or nasoantral window), although this is not considered a standard part of FESS (Fig. 3.23). Another twist that is sometimes performed during FESS is Bolgerization, which consists of stripping away part of the mucosa of the nasal septum in order to secure a loose middle turbinate and prevent lateralization.


Fig. 3.23
Nasoantral window. Coronal CT image shows surgical defects in the bilateral medial maxillary antrum walls (arrows) in addition to bilateral partial ethmoidectomies
3.11 FESS Complications
3.11.1 Discussion
Surgical packing material, such as gauze, may sometimes be left temporarily in the sinuses after functional endoscopic surgery for hemostasis. In the early postoperative period, the gauze can appear as heterogeneous material often containing foci of trapped air, but over time the retained gauze resembles a soft tissue mass with an attenuation of approximately 50 HU on CT (Fig. 3.24). Not all types of gauze packing contain radioattenuating markers. Furthermore, the retained packing may not show enhancement on CT or MRI. Occasionally, patients may not return for postsurgical follow-up, and the packing may remain for long periods of time, resulting in a gossypiboma. This may cause recurrence of sinus symptoms and may even predispose to infection. However, resorbable packing materials have also been developed that do not require removal.
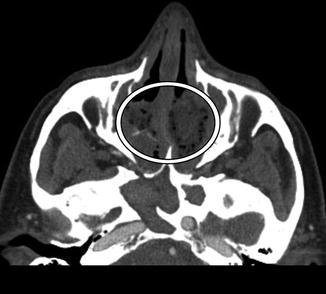

Fig. 3.24
Retained surgical packing (gossypiboma). The patient presents with headache after functional endoscopic sinus surgery a couple of weeks before and neglected to attend the routine postoperative appointment to have the packing removed. Axial CT image shows changes related to FESS and non-enhancing material that contains foci of air filling the ethmoid sinuses (encircled)
3.11.2 Discussion
Cephaloceles and cerebrospinal fluid leaks are serious complications of endoscopic sinus surgery that can result from inadvertently creating defects in the floor of the anterior cranial fossa. On CT, the presence of pneumocephalus is a helpful indicator that there is indeed intracranial penetration and cerebrospinal fluid leak (Fig. 3.25). High-resolution CT with multiplanar reconstructions is useful for evaluating the presence of bony dehiscence. However, the presence of sinus opacification contiguous with the intracranial compartment is suggestive, but not specific for encephalocele or meningocele. Rather, MRI is better suited for diagnosing meningoceles, encephaloceles, and associated soft tissue injury (Fig. 3.26). Radionuclide cisternographic studies do not adequately localize and characterize skull base defects well enough to be the sole diagnostic examination. Rather, radionuclide cisternography is reserved for complex cases when the diagnosis is in uncertain.
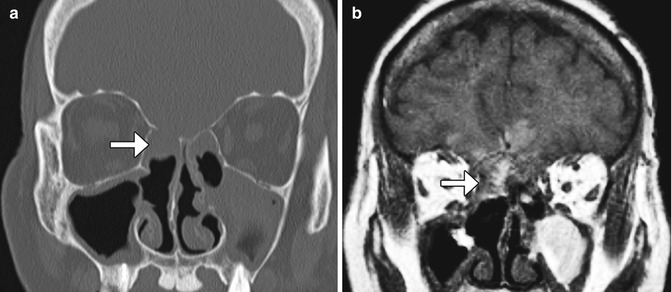

Fig. 3.25
Cerebrospinal fluid leak. The patient presented with headache and rhinorrhea after FESS. Coronal CT image shows left-sided pneumocephalus and a defect in the left cribriform plate

Fig. 3.26
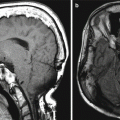
Encephalocele and intraparenchymal hemorrhage. Coronal CT image (a) shows internal ethmoidectomies and dehiscence of the right ethmoid roof (arrow). There is nonspecific opacification inferior to the dehiscence. Coronal (b) T1-weighted MRI shows herniation of brain tissue through the defect in the ethmoid roof. In addition, there is high signal intensity in a linear distribution (arrows), which corresponds to hemorrhage along the path of the misdirected surgical instrument
3.11.3 Discussion
Intraorbital complications related to FESS include herniation of intraorbital contents through iatrogenic defects in the lamina papyracea, orbital hemorrhage, optic nerve transection (Fig. 3.27), and orbital cellulitis. Intraorbital hemorrhage can result from direct injury to orbital vessels, ethmoid arteries, or extension into the orbit through a medial wall defect and may cause an acute rise in orbital pressure with rapid onset of proptosis and loss of vision. Orbital CT and MRI are both suitable modalities for evaluating orbital trauma related to FESS.
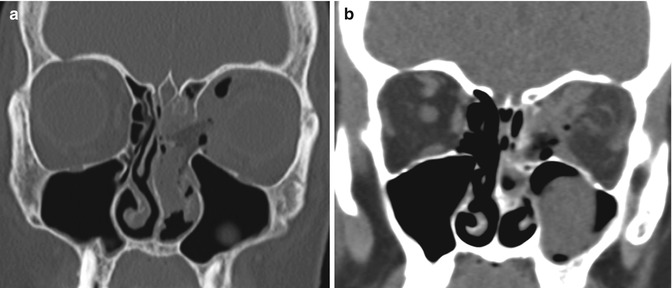

Fig. 3.27
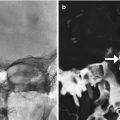
Orbital injury. The patient presented with left vision loss after FESS. Coronal CT images in the bone (a) and soft tissue (b) windows show a large defect in the left lamina papyracea, abundant pneumo-orbit, retrobulbar hemorrhage, and deformity of the optic nerve on the left side
3.11.4 Discussion
Both extracranial and intracranial vessels can be injured during FESS. The anterior ethmoidal arteries are particularly susceptible to laceration. Although this can be treated by clipping during the procedure (Fig. 3.28), the artery can potentially retract into the orbit, resulting in intraorbital hemorrhage. High-resolution CT can accurately detect the site of entry, which is usually via the fovea ethmoidalis or roof of the ethmoid sinus. Cerebral angiography is recommended to locate an associated pseudoaneurysm, which can often be treated endovascularly (Fig. 3.29).
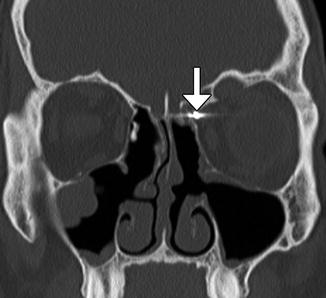
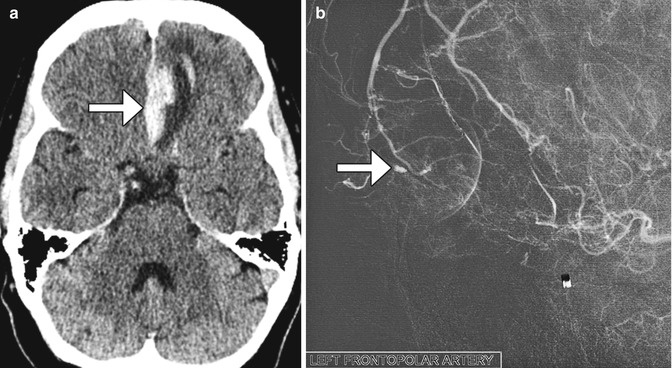

Fig. 3.28
Ethmoid artery injury. Coronal CT image shows a vascular clip (arrow) in the region of the left anterior ethmoid artery groove, which was applied to stop bleeding from the injury artery. There is evidence of left-sided internal ethmoidectomy with the presence of bony defect within the left lamina papyracea

Fig. 3.29
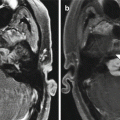
Anterior cerebral artery pseudoaneurysm. The patient presented with sudden-onset mental status changes and headache a few days after undergoing polypectomy. Axial CT image (a) shows left gyrus rectus intraparenchymal hemorrhage with a flame-shaped configuration (arrow). Digital subtraction cerebral angiogram (b) reveals a pseudoaneurysm (arrow)
3.11.5 Discussion
Medial bowing of the lamina papyracea can occur following internal ethmoidectomy perhaps due to the loss of internal structural support and forces of contracture during the postoperative healing period (Fig. 3.30). On average, the interorbital distance decreases by about 1 mm after surgery and mild enophthalmos results. These changes are usually subclinical.
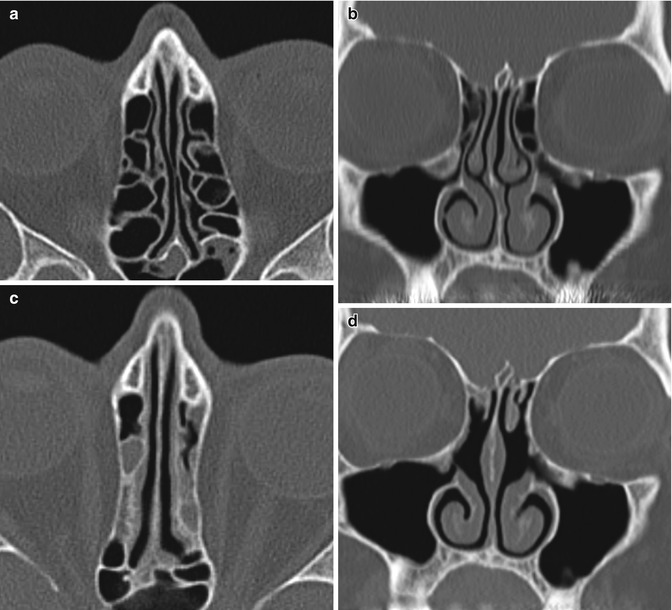

Fig. 3.30
Medialized lamina papyracea. Preoperative axial (a) and coronal (b) CT images show normal alignment of the bilateral lamina papyracea. Postoperative axial (c) and coronal (d) CT images show bilateral internal ethmoidectomies and middle turbinate with medial bowing of the laminae papyracea. There is also new opacification of the ethmoid sinuses
3.11.6 Discussion
Mucosal inflammatory disease is common after FESS (Fig. 3.31). Mucosal thickening up to 3 mm is considered normal. However, the degree of mucosal thickening demonstrated on imaging does not necessarily correlate with recurrent symptoms. Mucosal inflammation and synechiae are often indistinguishable on imaging and may certainly coexist. However, synechiae can appear as thin bands of soft tissue within or adjacent to the operative sinonasal cavity. These are most common after frontal sinusotomy.
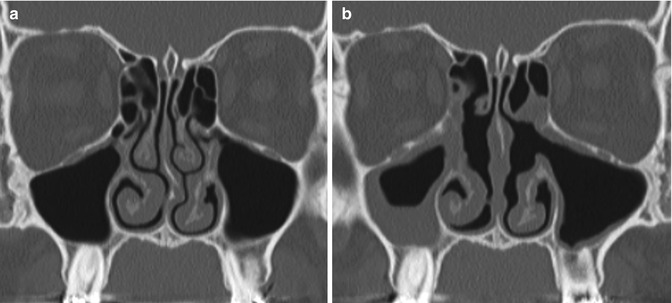

Fig. 3.31
Mucosal inflammation. Preoperative coronal CT image (a) shows mild mucosal thickening. Postoperative CT image (b) shows diffusely increased mucosal thickening, particularly in the right maxillary sinus after bilateral uncinectomy, as well as correction of septal deviation
3.11.7 Discussion
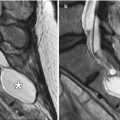
Mucoceles can form in previously operated sinuses due to blockage by the bone and/or scar tissue. Mucoceles are characterized by expansion of the sinus (Fig. 3.32). Although the absence of air within the affected sinus is sine qua non for mucoceles in nonoperated patients, this is not necessarily the case for postoperative mucoceles. Postoperative scar tissue may isolate a portion of the sinus, forming a compartment where the mucocele can form. This is sometimes termed “surgical ciliated cyst.”
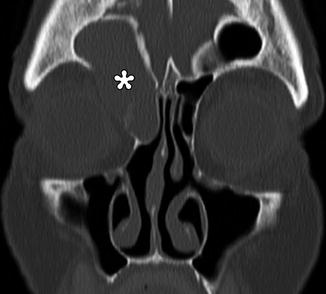

Fig. 3.32




Mucocele. Coronal CT image shows a right frontoethmoid mucocele (*) following frontal sinusotomy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree