
Improved TIPS Patency with Covered Stents
Over the past three decades, the transjugular intrahepatic portosystemic shunt (TIPS) has both fascinated and frustrated a generation of interventional radiologists. Although brilliant in concept, and highly effective in the immediate relief of portal venous hypertension, the procedure has been plagued since its inception by early shunt occlusion and clinical failure.
Rösch et al1 described the essential features of the TIPS in 1969, using a detachable Teflon tube to create a fistula between hepatic and portal veins in a dog model. In subsequent work with animals and human cadavers, Rösch and co-workers2 characterized the TIPS technique as “relatively simple,” but they found patency of the in vivo shunts to be limited to a matter of hours by thrombosis or migration of the tube segments.
Later authors3,4 attempted various methods of creating stable intrahepatic portosystemic fistulae, including cryotherapy of the tract and repeated or prolonged balloon dilatation. Colapinto et al5 used the latter technique in performing the first limited, TIPS series in humans. In nearly all cases, however, recoil of liver parenchyma rapidly occluded the unsupported shunts. Palmaz and colleagues,6 in 1985, overcame hepatic recoil by implanting metallic endovascular stents in the shunts of dogs, and it appeared that TIPS durability had finally been achieved. Richter et al7 placed the first stented TIPS in a human patient in 1989, and the procedure has since been performed in many thousands of patients worldwide.
Despite the rapid assimilation of stented TIPS into clinical use, primary shunt patency continues to hobble the technique. Although TIPS patency is no longer limited to hours or days, numerous large studies have reported shunt dysfunction in up to 85% of patients within one year.8–11 These patients, fortunately, are rarely symptomatic: recurrent variceal bleeding is seen in just 25% of patients with anatomic TIPS stenosis.8,12 Furthermore, stenotic or occluded TIPS shunts are readily treated by balloon angioplasty or additional stent placement, and these interventions can achieve longterm assisted patency rates of greater than 90%. Such efforts are costly, invasive, and require careful monitoring of the shunt by Doppler ultrasound or venography.13–15 Nonetheless, after 30 years of experience, TIPS still must be viewed as a temporary or multistage procedure.
The processes leading to TIPS dysfunction have been studied in detail, with no fewer than eight publications devoted to TIPS histology.16–23 These examinations have identified an underlying set of pathophysiological processes that might be prevented by applying a semi-permeable covering to the stent in the intrahepatic tract and draining hepatic vein. With this control achieved, long-term primary TIPS patency at last may be feasible. This chapter addresses the anatomy, physiology, and histology of TIPS and both the potential and realized impacts of TIPS stent-grafts.
 TIPS Dysfunction: Definition and Etiology
TIPS Dysfunction: Definition and Etiology
TIPS dysfunction must be distinguished from shunt-related complications. The latter, which also are seen after surgical shunting, include hepatic encephalopathy, right-sided heart failure, and deterioration of hepatic function.8–10 These outcomes are ironically reflective of a TIPS that is working “too well,” shunting blood from the portal system at a rate in excess of that which can be tolerated by the patient. This chapter considers the opposite situation: loss of shunt patency with inadequate shunt function.
In keeping with the classical surgical literature, primary patency is defined as the period from TIPS placement to shunt occlusion or the development of a significant stenosis. By the most stringent morphologic and hemodynamic criteria, a significant stenosis is one that reduces the shunt diameter by 50%, increases the portosystemic gradient above 15 mm Hg, or causes decreased flow velocity by Doppler ultrasound, even if the initiating symptoms do not recur. Assisted patency is the period between initial shunt revision (by whatever means) and irreversible occlusion of the shunt. The period of assisted patency may encompass numerous revisions.
The etiology of TIPS dysfunction correlates strongly with postprocedure time course.12–14 Immediate occlusion is frequently the result of technical error. Most often, the stent is too short or is malpositioned, failing to bridge the entire parenchymal tract. Short-term failure (hours to days) usually is caused by acute thrombosis and may be associated with biliary injury (see later). Mid- and long-term dysfunctions, by comparison, are almost always the result of progressive stenosis within the parenchymal tract or the recipient hepatic vein. Such stenoses are an integral part of the biological response to TIPS and are not under the direct control of the operator. It is by preventing or containing these responses that stent–grafts may serve to increase the primary patency of TIPS.
 Anatomic and Physiologic Considerations
Anatomic and Physiologic Considerations
The TIPS procedure has many features in common with other endovascular procedures. By disrupting the native vascular environment, angioplasty or stent placement incites a multifactorial process involving both physical and biological events. The normal response of arteries and veins to such perturbations has been studied extensively, and the prevailing theory can be summarized as follows: (1) injury to vascular endothelium occurs during dilatation; (2) the resulting surface irregularity and exposure of basement tissues induce platelet deposition; (3) platelet-derived growth factor (PDGF) and injury-mediated reparative processes induce the migration of synthetic smooth-muscle cells (SMCs) into the thrombus; (4) the SMCs elaborate a protein matrix, creating an organized, stable neointima; and (5) the neointima is covered by a smooth layer of endothelium.24–26 The eventual thickness of the neointimal layer varies directly with the degree of vascular injury and initial thrombus deposition.27–31 When excessive, the process is known as neointimal hyperplasia (NH).
Stent placement further alters the endovascular environment and may increase NH.28,32 As foreign material, exposed stents are a site for platelet aggregation. Stent struts at the luminal aspect of the vascular wall also disturb laminar flow, creating eddy currents that further enhance platelet deposition.33,34 The ongoing radial force generated by stents may exaggerate and prolong injury-mediated responses. In addition, the presence of a stent reduces vascular wall compliance. Some authors have suggested an inverse relationship between wall compliance and NH development, but the issue is debated in the literature.35–39
The TIPS shunt is an anatomically unique structure (Fig. 6–1), and physiologic responses occurring within it vary significantly by location. In the portal and hepatic veins, the TIPS extends into an essentially normal vascular structure. The parenchymal tract, by contrast, is an internally scaffolded hepatic laceration with no native vascular endothelium. At both ends of the parenchymal tract, the stent passes through the disrupted vessel wall and crosses the vascular lumen. An understanding of the processes in each area of the shunt is imperative to the successful application of TIPS stent–grafts.
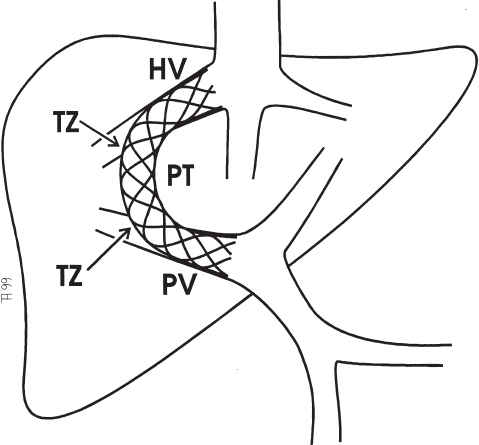
FIGURE 6–1. Schematic diagram of a TIPS, localizing the four physioanatomic regions: portal vein (PV), hepatic vein (HV), transition zones (TZ), and parenchymal tract (PT).
Portal Vein
At the portal end of a TIPS, interactions between the stent and vein are the dominant feature. The stent produces continuous radial force against the vein wall, reducing portal vein compliance and presenting an irregular foreign surface. Little endothelial disruption occurs, however, and vascular injury is insignificant. NH in the portal vein is consequently minimized, and the stent is rapidly covered by a thin, smooth neointima. Hemodynamically significant stenoses in the portal vein are exceedingly rare, occurring in fewer than 1% of patients.12
Hepatic Vein
Within the recipient hepatic vein, the usual responses described in the preceding are compounded by vascular trauma and by nonphysiologic hemodynamic factors. The hepatic vein may experience significant injury during TIPS creation, especially if multiple needle passes are required to establish portal access. In addition, the vessel may be smaller in native diameter than the final shunt, necessitating overdilatation of the vein. Once established, a TIPS significantly elevates blood flow velocity, volume, and pressure in the hepatic vein. Furthermore, inflow is directed toward the superior aspect of the vein above the shunt, resulting in a “jet effect” against the vessel wall. This small but continuous jet may result in mild but chronic injury. As a result of these factors, hemodynamically significant stenoses in the recipient hepatic vein are common, and their frequency increases with time (Fig. 6–2).
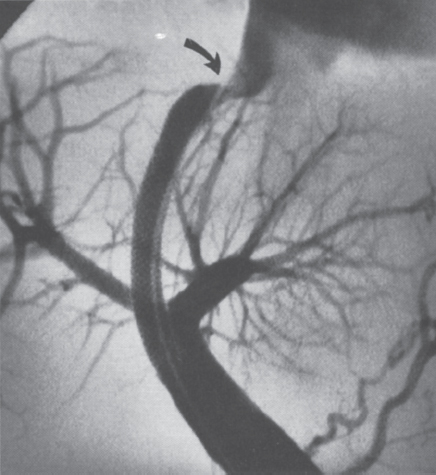
FIGURE 6–2. Stenosis at the hepatic venous outflow (arrow) in an otherwise patent TIPS.
Transition Zones
As the TIPS stent exits the parenchymal tract and enters the portal and hepatic vein, it arches across the vascular lumen like a sieve. Examinations of TIPS stents and other stents placed across vascular branch-points show a thin layer of neointima on the transluminal stent mesh, with flow maintained through the interstices (Fig. 6–3).40,41 A classical TIPS, therefore, is a partial shunt: Blood can flow through the TIPS or, by passing through the open mesh of the bare stent, through the normal course of the portal or hepatic veins. The degree of shunting varies inversely with the portosystemic gradient. Hemolysis can be seen in some cases, with cellular elements damaged by the mesh, but it is uncommon for this process to reach clinical significance.42
Parenchymal Tract
In contrast to venous portions of the shunt, where endothelial disruption is minimal, blood flowing through the parenchymal tract is exposed on all sides to bare, severely injured liver parenchyma. The coagulation cascade is strongly activated. In addition, liver parenchyma protrudes irregularly through the interstices of the stent, reducing luminal diameter and creating an undulating surface that may further disrupt laminar flow.16,19,21,43 These factors, possibly exaggerated by minimal wall compliance in the fibrotic liver, may increase the initial deposition of thrombus in the parenchymal portion of the shunt. As indicated, greater thrombus deposition is associated with a more exuberant proliferative response. Because the parenchymal tract has no natural intimal covering, the reactive covering of the tract is referred to as pseudointima, rather than neointima.

FIGURE 6–3. Longitudinal section of a TIPS stent at the hepatic vein transition zone. Note incomplete cellular coverage of the stent, with preservation of the native vein lumen. The triangular area of dark material represents bile staining in the parenchymal tract.
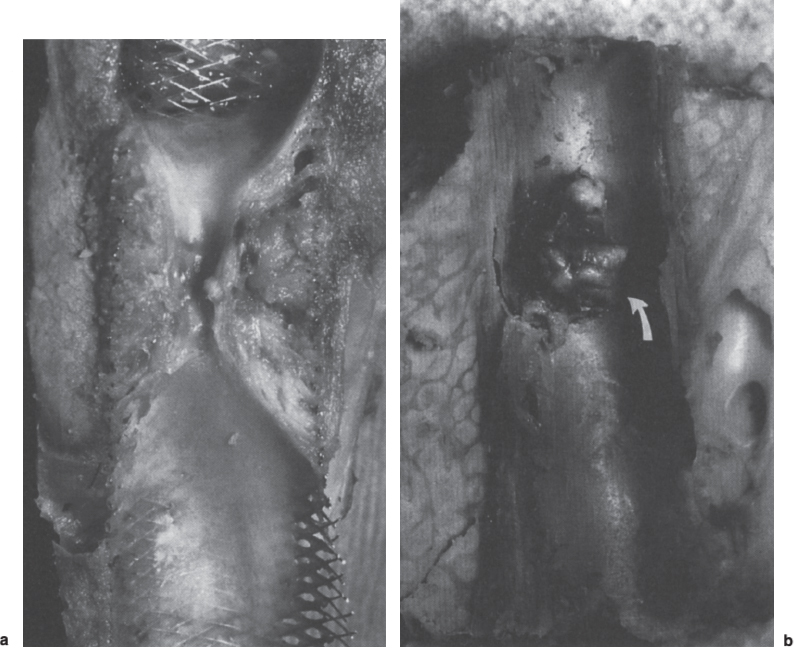
FIGURE 6–4. Longitudinal sections showing parenchymal tract stenosis: (a) diffuse narrowing involving the entire tract; (b) focal nodular stenosis (arrow) in a patient with a bile leak (not shown)
A high percentage of TIPS stenoses occur in the parenchymal tract, usually in the midterm (from a few days to 6 months) (Fig. 6–4). In the earlier period, the offending material is thrombus, evolving in a continuum to hyperplastic pseudointima in the later period. The distribution of material may be diffuse or strikingly focal. Interestingly, if thrombus or pseudointimal hyperplasia recurs after successful treatment of these stenoses by angioplasty or stent placement, the recurrence location in more than 80% of patients is identical to that seen initially (Fig. 6–5).12 In such cases, patients may require frequent revisions of the same site. Although the specific nature of this process has not been elucidated definitively, many investigators suspect that biliary duct injury is a contributing factor.
 The Effect of Biliary Secretions
The Effect of Biliary Secretions
In addition to exposing bare liver parenchyma, the TIPS procedure creates macroscopic and microscopic biliary–vascular fistulae. Several investigators have shown gross biliary-to-TIPS communication by using venography, cholangiography, or a specially designed dual-occlusion balloon (Fig. 6–6). Bile staining is commonly identified during the gross evaluation of explanted TIPS,17–23 and histologic examination often identifies encroachment of metaplastic biliary endothelium into the pseudointimal covering of the parenchymal tract.18,19,44 In some cases, the biliary endothelium forms neoducts or cysts. In others, it spreads through the pseudointima to form a biliary endothelial lining on the luminal surface of the TIPS shunt (Fig. 6–7). The endothelium is functional despite its ectopic location and releases biliary secretions into the shunt.
Laberge et al,17 in 1993, were first to suggest a correlation between biliary extravasation and pseudointimal proliferation. In a study of five stenotic or occluded TIPS, they identified transected bile ducts and bile-stained pseudointima in three cases (Fig. 6–8). They attributed the shunt failures to pseudointimal hyperplasia and suggested that the hyperplasia was an inflammatory response stimulated by bile.
Subsequent reports have supported the relationship between biliary injury and pseudointimal hyperplasia. Jalan et al,19 studied 34 TIPS patients and performed percutaneous biopsies of the pseudointima of eight patients with stenoses greater than 70%. Four of these samples had bile staining, and two demonstrated incorporated biliary endothelium. Histologic study of 10 explanted livers revealed four with mild or severe (>70%) stenosis. Biliary transactions and bile staining were present in all four cases, and the authors reported a correlation between the degree of stenosis and the size of the transected duct. No stenoses were seen in the absence of bile staining. The authors agreed with Laberge et al17 that an inflammatory response induced by bile was a significant contributing factor in shunt failure.
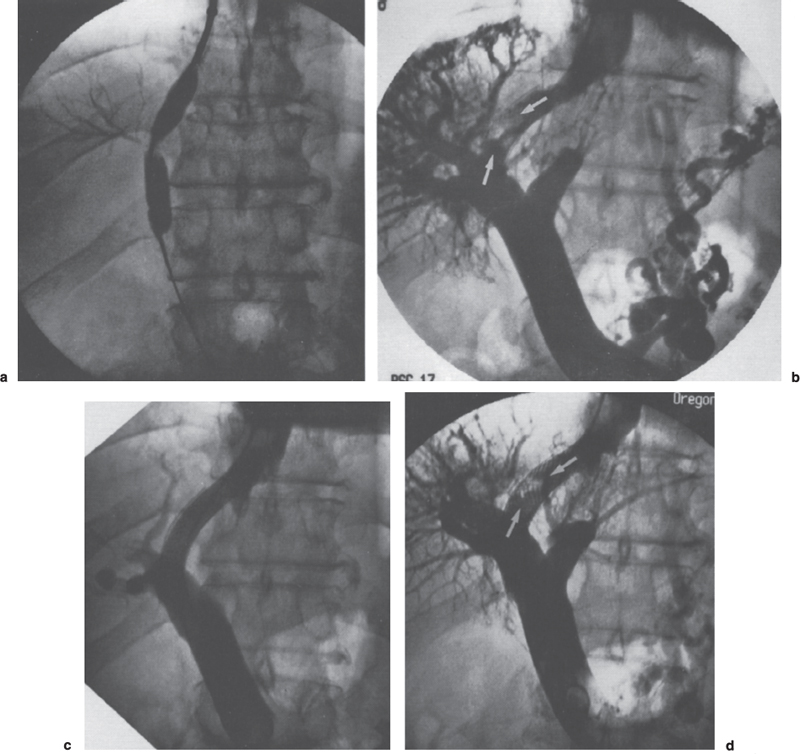
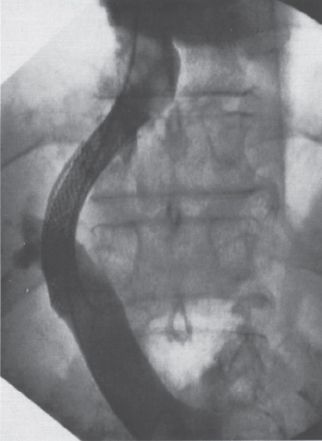
FIGURE 6–5. Relationship between a bile leak and TIPS dysfunction: (a) a bile leak was present at the time of initial TIPS creation but was not recognized; (b) the patient returned with persistent ascites 3 months later and was found to have a focal stenosis at the site of biliary injury (arrows); (c) placement of a second stent gave an excellent angiographic result and reduced the portosystemic gradient from 23 to 12 mm Hg; (d) an identical restenosis (arrow) was present one month later, on venography performed after abnormal ultrasound; (e) a covered stent-graft was placed, and the patient has remained patent over the subsequent follow-up period of nine months.
Saxon et al18 reported similar findings but offered a slightly different explanation. These authors described comparative studies of TIPS histology in 13 swine and 21 human patients. Severe shunt compromise (occlusion or stenosis greater than 75%) was identified within the parenchymal tracts of nine swine and eight human shunts. “Substantial” biliary fistulae were present in seven of the stenotic shunts from each group (77% and 88%, respectively). The associated pseudointimal hyperplasia was notable for invasion by metaplastic bile ducts containing active, mucin-secreting goblet cells (Fig. 6–9). In contrast, no evidence of significant biliary communication was found in two porcine and 13 human shunts that were widely patent at follow-up. The authors concluded that biliary duct injury was strongly associated with abnormalities in the parenchymal tract and with TIPS failure. Unlike the groups of LaBerge and Jalan,17,19 however, Saxon and colleagues18 suggested that the causative agent of shunt failure might be mucin, which is highly thrombogenic45,46 and postulated that exuberant mucin-induced thrombus might provide a physically larger matrix for subsequent ingrowth and hyperplasia of granulation tissue.
The mucin theory was supported by Teng et al.22 In an in vitro study, these researchers exposed cultured aortic SMCs to varying concentrations of bile and found that SMC activity (as measured by the production of DNA and protein) was significantly decreased at bile concentrations of 1% or greater. With concentrations of 2.5% or greater, 100% SMC mortality was observed within 3 days. In a concurrent in vivo study in 45 swine, the group found no significant correlation between bile leaks (seen in 13 animals) and neointimal thickness within the involved shunt. The authors concluded that bile does not cause NH and may in fact be protective against the process. Like Saxon et al, Teng and associates implicated mucin as the responsible agent.
 The Role of Stent–Grafts
The Role of Stent–Grafts
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



