(1)
Department of Nuclear Medicine, Kuwait University, Safat, Kuwait
Abstract
Inflammation (described as early as 3000 BC in an Egyptian papyrus [1]) is still a common problem despite continuous advancements in prevention and treatment methods. The delineation of the site and extent of inflammation is crucial to the clinical management of infection and for monitoring the response to therapy [2]. Nuclear medicine along with morphological imaging has an important role in achieving this goal.
4.1 Definition
Inflammation (described as early as 3000 BC in an Egyptian papyrus [1]) is still a common problem despite continuous advancements in prevention and treatment methods. The delineation of the site and extent of inflammation is crucial to the clinical management of infection and for monitoring the response to therapy [2]. Nuclear medicine along with morphological imaging has an important role in achieving this goal.
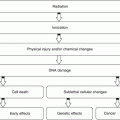
Inflammation is a complex tissue reaction to injury. Injury may be caused not only by microbes but also by chemical, physical, immunological, or radiation agents:
Physical agents
Trauma
Heat
Chemical agents
Chemotherapy
Industrial accidents
Immunological agents
Antigen-antibody reactions
Radiation
Radiation therapy
Nontherapeutic radiation exposure
Microbes
Bacteria
Viruses
Fungi
Inflammation is fundamentally a protective reaction against the causes of cell injury as well as the consequence of such injury. However, inflammation is potentially harmful and may even be life threatening. Since most of the essential components of the inflammatory process are found in the circulation, inflammation occurs only in vascularized tissue. Inflammation is generally considered a nonspecific response, because it happens in the same way regardless of the stimulus and the number of exposures to the stimulus [2].
4.2 Classification of Inflammation
Inflammation may be classified as acute or chronic. Acute inflammation is the immediate or early response to injury and is of relatively short duration. It lasts for minutes, hours, or at most few days. Chronic inflammation, on the other hand, is of longer duration and may last from weeks to years [3]. The distinction between acute and chronic inflammation, however, depends not only on the duration of the process but also on other pathological and clinical features.
4.3 General Pathophysiological Changes of Inflammation
4.3.1 Local Pathophysiological Changes of Inflammation
4.3.1.1 Acute Inflammation
Acute inflammation continues only until the threat to the host has been eliminated, which usually takes 8–10 days, although this is variable. Inflammation is generally considered to be chronic when it persists for longer than 2 weeks [2]. Many regional and systemic changes accompanying acute inflammation are mediated by certain chemicals produced endogenously called chemical mediators and are behind the spread of the acute inflammatory response following injury to a small area of tissue into uninjured sites. These chemical mediators include mediators released from cells such as histamine and prostaglandins and others in plasma which are released by the systems contained in the plasma. These are the four enzymatic cascade systems, namely, the complement system, the kinins, the coagulation factors, and the fibrinolytic system, which produce several inflammatory mediators [4–6]. Table 4.1 summarizes the main chemical mediators of inflammation. Acute inflammation is characterized by the following major regional components.
Table 4.1
Chemical mediators of inflammation
Mediator | Characteristics and role in inflammation |
A. Cell factors | |
Histamine | Stored in mast cells, basophil and eosinophil leukocytes, and platelets |
Release from sites of storage is stimulated by complement components C3a and C5a, and by lysosomal proteins released from neutrophils | |
Responsible for vasodilatation and the immediate phase of increased vascular permeability | |
Lysosomal compound | Released from neutrophils and includes cationic proteins, which may increase vascular permeability, and neutral proteases, which may activate complement |
Prostaglandins | Long-chain fatty acids derived from arachidonic acid and synthesized by many cell types. Some prostaglandins potentiate the increase in vascular permeability caused by other compounds |
Leukotrienes | Synthesized from arachidonic acid, especially in neutrophils, and have vasoactive properties |
5-Hydroxytryptamine (serotonin) | A potent vasoconstrictor present in high concentrations in mast cells and platelets |
Lymphokines | Released by lymphocytes and may have vasoactive or chemotactic effects |
B. Plasma factors | |
Products of complement activation: | |
C5a | Chemotactic for neutrophils; increases vascular permeability; releases histamine from mast cells |
C3a | Similar to but less active than C5a |
C567 | Chemotactic for neutrophils |
C56789 | Cytolytic activity |
C4b, 2a, 3b | Facilitates phagocytosis of bacteria by macrophages (opsonization of bacteria) |
Kinin system | Bradykinin included in the system is the most important vascular permeability factor, also a mediator for pain which is a major feature of acute inflammation |
Coagulation factors | Responsible for the conversion of soluble fibrinogen into fibrin, a major component of the acute inflammatory exudate |
Fibrinolytic system | Plasmin included in the fibrinolytic system is responsible for the lysis of fibrin into fibrin degradation products, which have a local effect on vascular permeability |
4.3.1.1.1 Local Vascular Changes
1.
Vasodilation following transient vasoconstriction is one of the most important changes that accompany acute inflammation, and it persists until the end of the process. It involves first the arterioles and then results in the opening of new capillary beds in the area.
2.
Increased vascular permeability due to:
Contraction of endothelial cells with widening of intercellular gaps.
Direct endothelial injury, resulting in endothelial cell necrosis and detachment.
Leukocyte-mediated endothelial injury: Leukocytes adhere to the endothelium, which becomes activated, thereby releasing toxic oxygen species and proteolytic enzymes and causing endothelial injury.
Angiogenesis: Endothelial cells may proliferate and form new capillaries and venular beds. These capillary sprouts remain leaky until endothelial cells differentiate.
3.
Stasis (slowing of circulation) resulting from increased permeability with extravasation of fluid into the extravascular spaces causing the concentration of red blood cells in the small vessels and increased viscosity of blood and slowing of circulation in the local vessels
4.3.1.1.2 Formation of Exudate
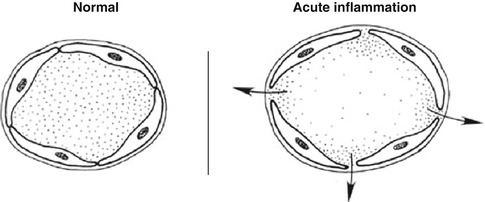
Increased permeability of the microvasculature, along with the other changes described, leads to leakage of “exudate,” an inflammatory extravascular fluid with a high protein content and cellular debris with a specific gravity of above 1.020. This is the hallmark of acute inflammation, which may also be called exudative inflammation. Exudate varies in composition. In early or mild inflammation, it may be watery (serous exudate) with low plasma protein content and few leukocytes. In more advanced inflammation, the exudate becomes thick and clotted (fibrinous exudate). When large numbers of leukocytes accumulate (Fig. 4.3), the exudate consists of pus and is called suppurative, while if it contains erythrocytes due to bleeding, it is referred to as hemorrhagic. Pus, accordingly, is a variant of exudate that is particularly rich in leukocytes, mostly neutrophils and parenchymal cell debris. Exudate should be differentiated from “transudate,” which is a fluid with low protein concentration and a specific gravity of less than 1.012. Transudation is associated with normal endothelial permeability [3, 4].
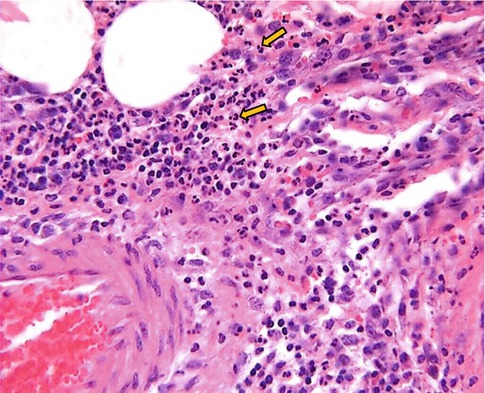

Fig. 4.3
Microphotograph of acute inflammation showing numerous inflammatory cells particularly polymorphonuclear leukocytes
4.3.1.1.3 Local Cellular Events
1.
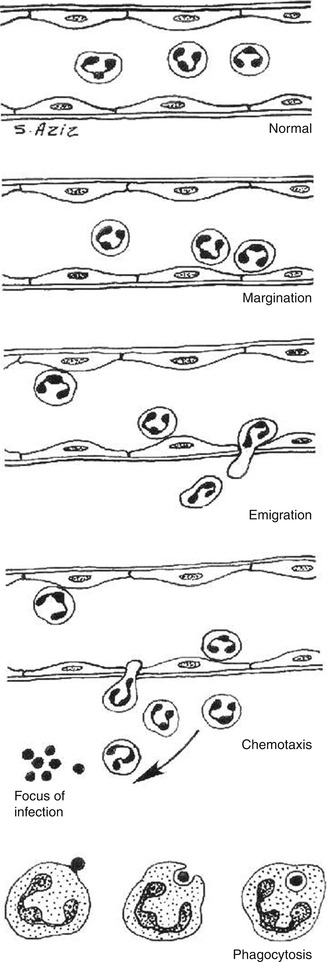
Margination
After stasis develops, leukocytes will be peripherally oriented along the vascular endothelium, a process called leukocytic margination (Fig. 4.2).
2.
Emigration (diapedesis)
Leukocytes emigrate from the microcirculation and accumulate at the site of injury.
3.
Chemotaxis
Once outside the blood vessel, the cells migrate at varying rates of speed in interstitial tissue toward a chemotactic stimulus in the inflammatory focus. Granulocytes, including the eosinophils, basophils, and some lymphocytes, respond to such stimuli and aggregate at the site of inflammation. The primary chemotactic factors include bacterial products, complement components C5a and C3a, kallikrein and plasminogen activators, products of fibrin degradation, prostaglandins, and fibrinopeptides. Histamine is not a chemotactic factor but facilitates the process. Some bacterial toxins, particularly from gram-negative bacteria and streptococcal streptolysins, inhibit neutrophil chemotaxis [2–4].
4.
Phagocytosis
This defense mechanism is particularly important in bacterial infections. The polymorphonuclear leukocytes and macrophages ingest debris and foreign particles.
4.3.1.2 Local Sequelae of Acute Inflammation
Acute inflammation has several possible local sequelae. These include resolution, suppuration (formation of pus), organization, and progression to chronic inflammation. Resolution means complete restoration of tissues to normal. Organization of tissues is their replacement by granulation tissue with formation of large amounts of fibrin, new capillaries growing into fibrin, macrophages migrating into the zone, and proliferation of fibroblasts resulting in fibrosis and the consequent organization of exudate.
4.3.1.3 Chronic Inflammation
Acute inflammation may progress to a chronic form characterized by reduction of the number of polymorphonuclear leukocytes and proliferation of fibroblasts with collagen production. Chronic inflammation may be primary with no preceding acute inflammatory reaction. Chronic inflammation, whether following acute inflammation or not, is characterized by a proliferative (fibroblastic) rather than an exudative response with predominantly mononuclear cell infiltration (macrophages, lymphocytes, and plasma cells) (Fig. 4.4). Abnormal vascular permeability is also common, but to a lesser extent than in acute inflammation with formation of new capillaries.
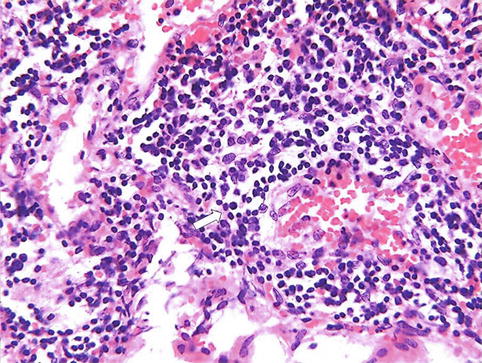

Fig. 4.4
Microphotograph of chronic inflammation illustrating the different types of inflammatory cells, the mononuclear cells including lymphocytes (arrow) and plasma cells (open arrow)
4.3.1.4 Abscess Formation
Abscess is defined as a collection of pus in tissues, organs, or confined spaces and usually caused by bacterial infection. The abscess formation starts by a phase of cellulitis, characterized by hyperemia, leukocytosis, and edema, without cellular necrosis or suppuration. This stage is also called phlegmon. It may be followed by necrosis, liquefaction, and formation of pyogenic membrane surrounding the pus, which results in abscess formation that can be present with both acute and chronic inflammation.
4.3.2 Systemic Pathophysiological Changes of Inflammation
Three major systemic changes are associated with inflammation: leukocytosis, fever, and an increase in plasma proteins. Leukocytosis is an increased production of leukocytes due to stimulation by several products of inflammation such as complement C3a and colony-stimulating factors. A febrile response is due to the pyrogens. The increase in plasma proteins is due to stimulation of the liver by some products of inflammation, leading to increased synthesis of certain proteins referred to as acute-phase reactants which include C-reactive protein, fibrinogen, and haptoglobin as anti-inflammatory [2].
4.3.3 Pathophysiological Changes of Healing
Healing of tissue after injury is closely linked to inflammation since it starts by acute inflammation. Healing may lead to restoration of normal structure and function of the injured tissue (resolution) or to the formation of a scar consisting of collagen (repair) when resolution is not achieved because the tissue is severely injured or cannot regenerate.
In either case, acute inflammation occurs first and for this reason is considered the defensive phase of healing. Healing (resolution and repair) occurs in two overlapping phases, reconstruction and maturation. The reconstructive phase starts 3–4 days after injury, continues for approximately 2 weeks, and is characterized by fibroblasts followed by collagen synthesis. The maturation phase is characterized by cell differentiation, scar formation, and remodeling of the scar; it begins several weeks after injury and may take up to 2 years to complete.
4.4 Pathophysiology of Major Soft Tissue Inflammation
4.4.1 Abdominal Inflammation
4.4.1.1 Abdominal Abscess
There are several types of abdominal infection: abscess; cellulitis (phlegmon), i.e., early inflammation of the soft tissue prior to or without formation of an abscess; and peritonitis. Abscesses can fall into three categories:
1.
Intraperitoneal abscess
2.
Retroperitoneal abscess
3.
Visceral abscess (hepatic, pancreatic, splenic)
The organisms causing abscesses may reach the tissue by direct implantation such as penetrating trauma and may spread from contiguous infection through hematogenous or lymphatic routes from a distant site or through migration of resistant flora into an adjacent, normally sterile area such as in perforation of an abdominal viscus. Factors predisposing to abscess formation include impaired host defense mechanisms, trauma/surgery, obstruction of urinary, biliary, or respiratory passages, foreign bodies, chemical or immunological irritation, and ischemia. Abdominal surgery (particularly of the colon, appendix, and biliary tree) and trauma are the most common; less common are appendicitis, diverticulitis, and pelvic inflammatory disease.
Management of these infections requires prompt recognition, early localization, and effective drainage, as well as appropriate antimicrobial use. Once the diagnosis is made and the abscess is localized, treatment should begin promptly. Localization is crucial since, for example, percutaneous drainage is inappropriate for abscesses in certain locations such as the posterior subphrenic space or in the porta hepatis [5].
Accumulation of leukocytes in the abscess is the pathophysiological basis for using labeled white blood cells for abscess imaging. In the acute phase, migration of leukocytes is vigorous. Later, the migration rate slows down and the cell type changes from predominantly neutrophils to mononuclear cells (lymphocytes, plasma cells, and macrophages). This pathophysiological change associated with the chronic state explains the better diagnostic accuracy of labeled leukocyte scans in acute as opposed to chronic abscesses.
Inflammatory Bowel Disease (IBD): IBD is an idiopathic disease, probably involving an immune reaction of the body to its own intestinal tract tissue. The two major types of IBD are ulcerative colitis (UC) and Crohn’s disease (CD) [6–8].
In UC, inflammation always begins in the rectum, extends proximally a certain distance, and then abruptly stops. A clear demarcation exists between involved and uninvolved mucosa. The rectum is always involved in UC and may remains confined to the rectum in approximately 25 % of cases. Pancolitis occurs in 10 % of patients. UC primarily involves the mucosa and the submucosa, with formation of crypt abscesses and mucosal ulceration. In severe UC, inflammation and necrosis can in rare cases extend below the lamina propria to involve the submucosa and the circular and longitudinal muscles. Crohn’s disease, on the other hand, consists of segmental involvement by a nonspecific granulomatous inflammatory process. The most important pathological feature is the involvement of all layers of the bowel, not just the mucosa and the submucosa, as is characteristic of UC. Furthermore, CD is discontinuous with skip areas interspersed between one or more involved areas [6–8] (see also Chap. 9).
4.4.2 Chest Inflammation
The chest is a common site of various types of infection, acute and chronic. Such infections are frequent in the elderly and in immunosuppressed patients, including cancer patients. Common inflammatory conditions relevant to nuclear medicine include pneumonia, sarcoidosis, diffuse interstitial fibrosis, and Pneumocystis (jiroveci) carinii pneumonia (see also Chap. 11).
4.4.2.1 Sarcoidosis
Sarcoidosis is an inflammatory condition of uncertain etiology characterized by the presence of noncaseating granulomas involving multiple organs. Current evidence points to a genetic predisposition and exposure to as yet unknown transmissible agent(s) and/or environmental factors as etiological agents [9]. The lung is most commonly and usually the first site of involvement. The inflammatory processes extend through the lymphatics to the hilar and mediastinal nodes. The lung is involved in more than 90 % of cases. Pulmonary sarcoidosis starts as diffuse interstitial alveolitis, followed by the characteristic granulomas. Granulomas are present in the alveolar septa as well as in the walls of the bronchi and pulmonary arteries and veins. The center of the granuloma contains epithelioid cells derived from mononuclear phagocytes, multinucleated giant cells, and macrophages. Lymphocytes, macrophages, monocytes, and fibroblasts are present at the periphery of the granuloma [10].
The diagnosis is based on a compatible clinical and/or radiological picture, histopathological evidence of noncaseating granulomas in tissue biopsy specimens, and exclusion of other diseases capable of producing similar clinical or histopathological appearances. Even microscopically, the noncaseating granulomas are not specific [9]. Infection by mycobacterial species other than Mycobacterium tuberculosis frequently leads to the production of noncaseating granulomas [11]. The disease runs a benign course with spontaneous remission of the activity though some degree of residual pulmonary function abnormality persists. Only a minority of patients develop complicated disease, which may lead to blindness, renal failure, liver failure, and heart involvement.
4.4.2.2 Pneumocystis carinii (jiroveci) Pneumonia
Pneumocystis carinii (jiroveci) pneumonia (PCP) is a condition that may be endemic or epidemic. It is caused by Pneumocystis carinii (jiroveci), which is a fungus. The condition is common in premature infants, debilitated children, and other immunocompromised conditions, particularly the acquired immune deficiency syndrome (AIDS). It is also seen in congenital immunodeficiency and in patients who are receiving chemotherapy and corticosteroids [12]. It is the most common infection in AIDS patients, and it remains an important cause of morbidity and mortality [13]. Transmission is usually airborne. The pathological changes are predominantly in the lungs with an inflammatory reaction consisting of plasma cells of variable amount, monocytes, and histiocytes. The diagnosis is established through identification of the organisms in bronchial secretions obtained by bronchoalveolar lavage or bronchial washings. Gallium-67 is an important imaging modality that helps in the diagnosis and evaluation of the activity of the disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree