(1)
Department of Medical Imaging, Linkou Chang Gung Memorial Hospital, Taoyuan, Taiwan
(2)
Department of Medical Imaging and Radiological Sciences, Institute of Radiological Research Chang Gung University, Taoyuan, Taiwan
11.1 Ketamine Cystitis
Case 1
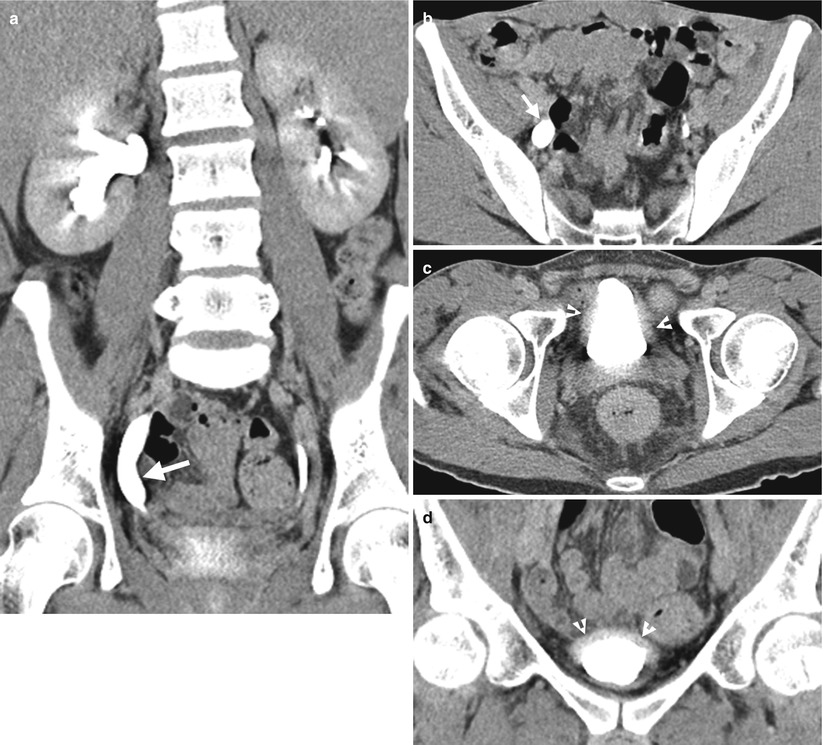
Fig. 11.1
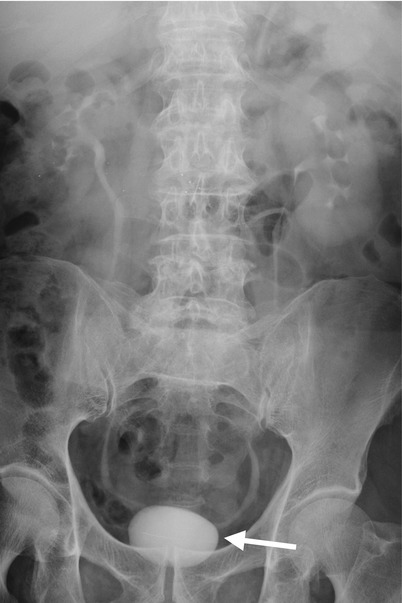
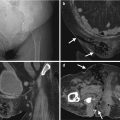
Computed tomography urography (CTU) shows urinary tract findings of a patient with ketamine abuse. Coronal enhanced CTU (Fig. 11.1a) shows mild right hydronephrosis and obvious hydroureter (arrow). Axial enhanced CTU (Fig. 11.1b) at SI joint level shows obvious dilatation of the right middle ureter (arrow). Axial (Fig. 11.1c) and coronal (Fig. 11.1d) CTU images at urinary bladder level show obvious wall thickening (arrowheads) of the urinary bladder. Note the small size of the urinary bladder.
Key Diagnostic Features
Ketamine abuse would cause interstitial cystitis [1], which on images are characterized by wall thickening of a very small-sized bladder. The patients with ketamine cystitis usually present with urgency, frequency, and dysuria, which is related with decreased bladder capacity [1]. Some patients with ketamine abuse may have unilateral or bilateral hydronephrosis or hydroureter in addition to cystitis [1]. Although a history of ketamine abuse is necessary to make a diagnosis, cystitis in a young adult or teenager is very unusual, and a high suspicion of ketamine-induced urological diseases should be raised especially in negative result of urine culture.
11.2 Flank Herniation After Nephrectomy
Case 2
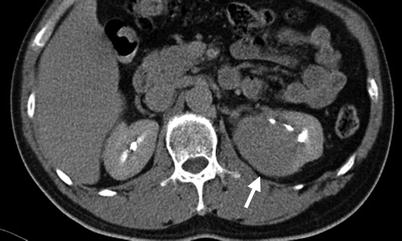
Fig. 11.2
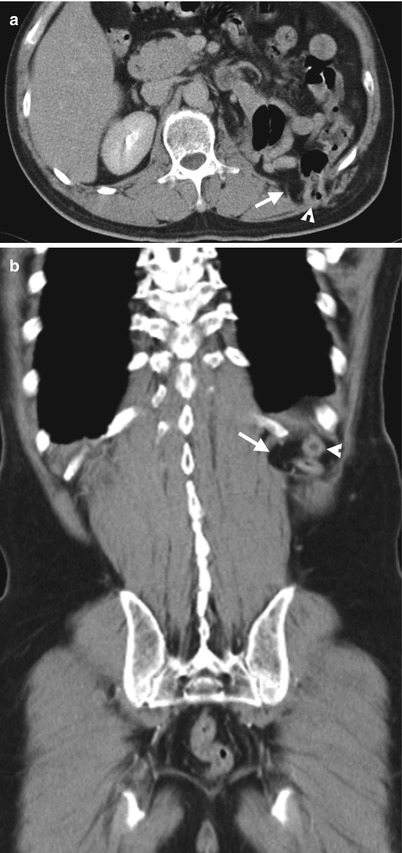
Fig. 11.3
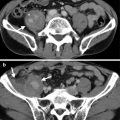
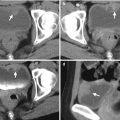
Computed tomography (CT) shows imaging findings of fat and colon herniation at the left flank after left nephrectomy. Enhanced renal CT image (Fig. 11.2) shows a left renal solid tumor (arrow). Left nephrectomy was done and a renal oncocytoma is diagnosed on histological examination. Axial (Fig. 11.3a) and coronal (Fig. 11.3b) images on enhanced follow-up CT 9 months after left nephrectomy show absence of left kidney in the left renal fossa due to left nephrectomy. Instead, small bowel loops and descending colon occupy the left renal fossa. Furthermore, left retroperitoneal fat (arrows) and descending colon (arrowheads) herniate through weakened wall of the left posterior flank.
Key Diagnostic Features
Flank herniation after nephrectomy is occasionally seen on images. It occurs as flank wall weakness after nephrectomy via retroperitoneal approach. Typically, retroperitoneal fat would herniate through the weakened flank wall area. By comparing the wall contour of the normal side, flank herniation on images could be easily identified. Sometimes, adjacent bowel loops also herniate though the weakened flank wall.
11.3 Colocutaneous Fistula After Nephrectomy
Case 3
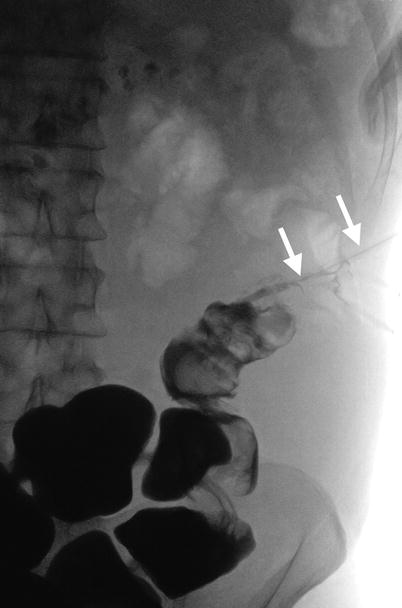
Fig. 11.4

Fig. 11.5
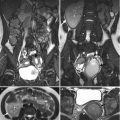
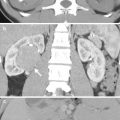
Fistulography and computed tomography (CT) show imaging findings of colocutaneous fistula of a patient who has undergone left nephrectomy for emphysematous pyelonephritis. Fistulography (Fig. 11.4) via a tube (arrows) inserted in the left flank shows opacification of the descending and sigmoid colon, suggestive of colocutaneous fistula. Contiguous enhanced coronal CT images (Fig. 11.5a, b) show a fistula tract (arrowheads) from the left flank skin to the descending colon. Note absence of the left kidney in the left retroperitoneum consistent with left nephrectomy.
Key Diagnostic Features
Colocutaneous fistula after nephrectomy is rare. Fistulography is useful to confirm communication of the skin and the colon. CT usually could detect the skin defect caused by a colocutaneous fistula; however, it is sometimes difficult to make sure of the colonic communication to the skin defect on CT.
11.4 Urothelial Carcinomas of the Ureteral Stump
Case 4
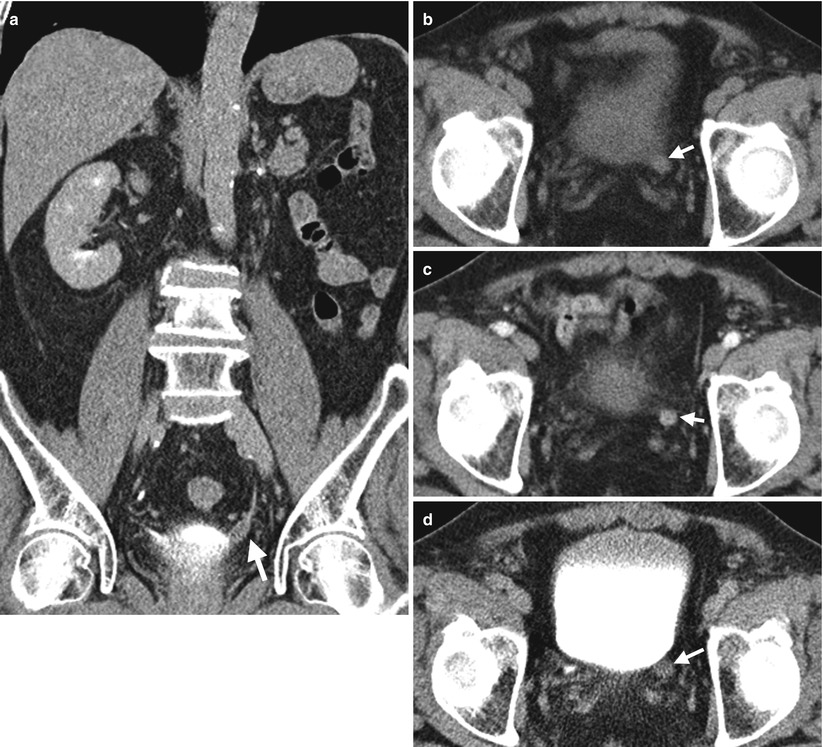
Fig. 11.6
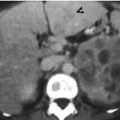
Computed tomography (CT) shows a urothelial carcinoma of the left ureteral stump. Figure 11.6a, enhanced coronal CT image of excretory phase shows abnormal soft tissue density of the left ureteral stump (arrow) extending to the left ureterovesical junction (UVJ). Absence of the left kidney in the left renal fossa is consistent with prior surgery of left nephroureterectomy. Figure 11.6b, unenhanced axial CT image shows a soft tissue nodule in the left distal ureter near the left UVJ. Figure 11.6c, enhanced axial CT image of arterial phase shows enhancement of the left ureteral stump with thickened wall appearance (arrow). Figure 11.6d, enhanced axial CT image of excretory phase shows abnormally enlarged size of the left ureteral stump, consistent with ureteral stump cancer, which is proved by subsequent surgery with histological evidence.
Case 5
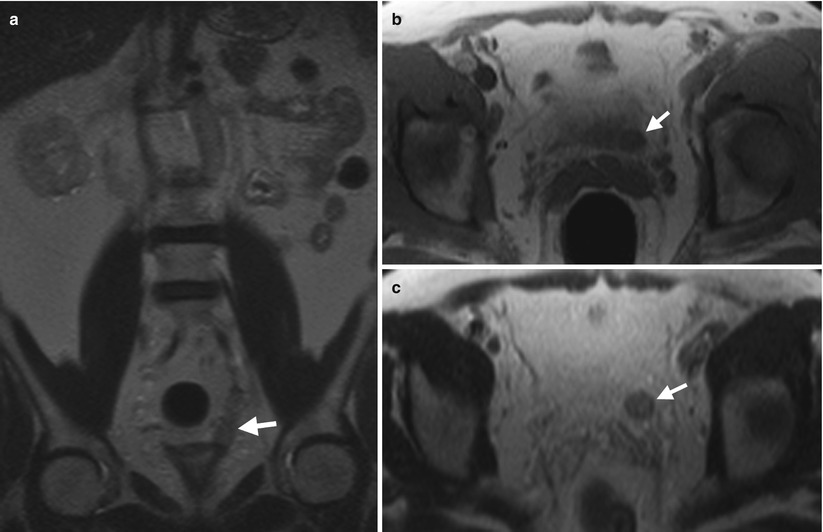
Fig. 11.7
Magnetic resonance imaging (MRI) shows a left ureteral stump cancer after left nephroureterectomy. Figure 11.7a, unenhanced coronal T2-weighted image (T2WI) of MRI shows an abnormally enlarged left ureteral stump (arrow). Figure 11.7b, unenhanced axial T1-weighted image (T1WI) of MRI shows the left distal ureteral stump appearing as a soft tissue mass (arrow). Figure 11.7c, unenhanced axial T2-weighted image of MRI, of 1 cm cranial to Fig. 11.7b level, shows intermediate signal intensity of the left distal ureteral stump mass (arrow), representing a neoplasm rather than a calculus. Histological examination of the surgical specimen of the left ureteral stump reveals a urothelial carcinoma.
Key Diagnostic Features
Urothelial carcinomas of the ureteral stump occur in the residual ureteral stump after nephroureterectomy. The standard surgery for urothelial carcinomas of the upper renal tract is nephroureterectomy with bladder cuff excision. However, the most distal part of the ureter to ureterovesical junction (UVJ) might remain unremoved by technical limitations hindering bladder cuff excisions. The remaining part of the ureter is called ureteral stump. The ability of tracing the ureteral stump on images is fundamental for diagnosing ureteral stump cancer, which could be traced from the UVJ to the remaining distal ureteral segment. On computed tomography or magnetic resonance image, urothelial carcinomas of the ureteral stump could be diagnosed by identifying abnormal enlargement of the ureteral stump occupied by a soft tissue mass with contrast enhancement.
11.5 Renal Subcapsular Hematoma After Extracorporeal Shock Wave Lithotripsy (ESWL)
Case 6

Fig. 11.8
Contiguous unenhanced renal CT images (Fig. 11.8a, b) of a patient undergoing ESWL for left renal stones 1 week ago show a large hematoma (arrows) of the left subcapsular space. The left kidney (arrowhead) is anteriorly displaced and stretched by the hematoma. There are residual stones in the left kidney showing high densities.
Case 7
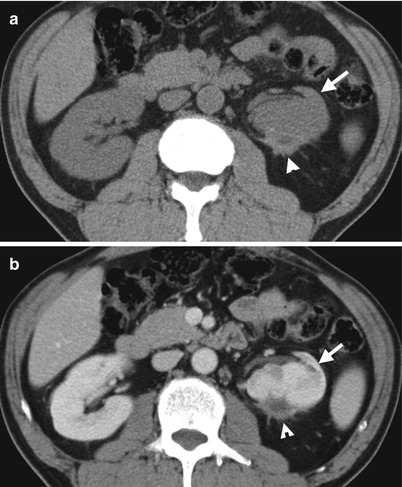
Fig. 11.9
Unenhanced (Fig. 11.9a) and enhanced (Fig. 11.9b) axial CT images show a chronic subcapsular hematoma (arrowheads) with adjacent fibrosis. The left kidney (arrows) is deformed and atrophic in appearance. The patient underwent ESWL 5 years ago, and left renal subcapsular hematoma is shown on several follow-up CT studies, including this one.
Case 8
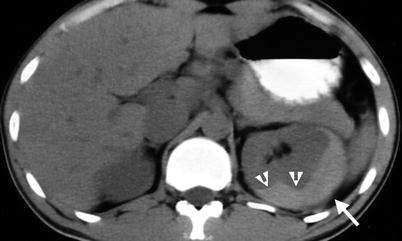
Fig. 11.10
Unenhanced computed tomography (Fig. 11.10) after ESWL for left renal stone shows high density acute hematoma (arrow) of left subcapsular space with mass effect deforming left renal contour (arrowheads).
Key Diagnostic Features
Renal subcapsular hematoma is a complication of ESWL [2]. It typically appears as a lenticular shape lesion with obvious mass effect on the adjacent renal parenchyma by a tight renal capsule. The ipsilateral kidney could be displaced by the hematoma; however, the compressed, deformed, and depressed renal parenchyma adjacent to the hematoma is conspicuous. Sometimes, subcapsular hematoma could cause hypertension by hemodynamic changes of ipsilateral kidney, which is called page kidney. Chronic subcapsular hematoma usually accompanies adjacent fibrosis and ipsilateral renal atrophy.
11.6 Renal Vascular Injury After Surgery or Intervention
Case 9
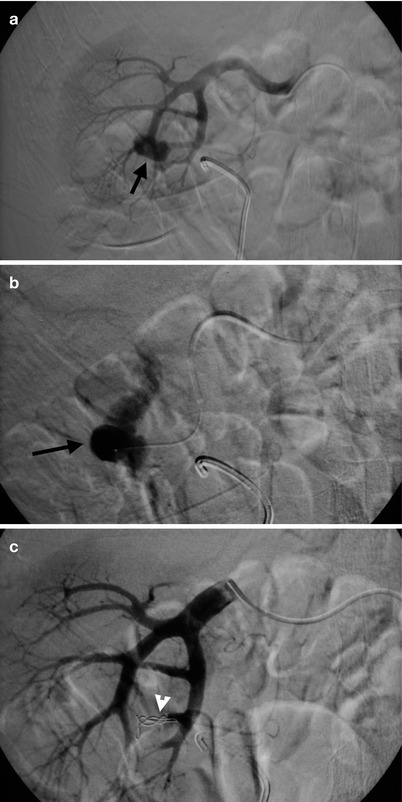
Fig. 11.11
Renal arteriography and transcatheter arterial embolization for the right renal artery were undertaken for a patient who has undergone renal bisection for staghorn stone. Right renal arteriography (Fig. 11.11a) shows an abnormal contrast medium pooling (arrow) at the right renal lower pole. Right renal arteriography with superselective catheterization of the bleeding vessel (Fig. 11.11b) shows obvious contrast extravasation (arrow). Right renal arteriography after embolization (Fig. 11.11c) shows disappearance of contrast pooling after embolization by coils (arrowhead) in the bleeding vessel.
Case 10
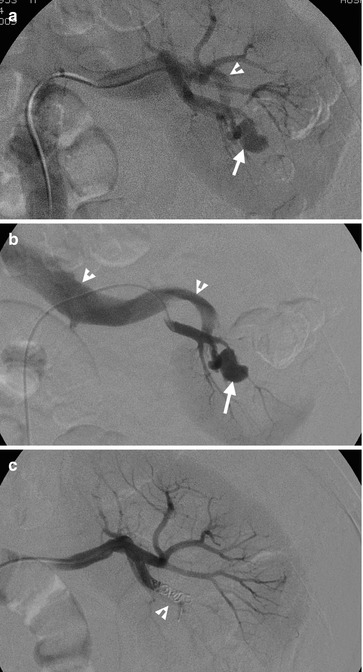
Fig. 11.12
Left renal arteriography (Fig. 11.12a) shows a pseudoaneurysm (arrow) at the left renal pole with early opacification of the left renal vein (arrowhead), indicating the presence of left arteriovenous fistula (AVF). Left renal arteriography with superselective catheterization of the left renal lower polar artery (Fig. 11.12b) shows direct and immediate opacification of the left renal vein (arrowheads) via left renal AVF and pseudoaneurysm (arrow). Left renal arteriography after embolization (Fig. 11.12c) shows no more opacification of pseudoaneurysm and AVF as well as absence of early opacification on the left renal vein. Coils (arrow) are deployed in the left renal lower polar artery for embolization. The patient has undergone right partial nephrectomy for a renal cell carcinoma before left renal arteriography.
Case 11
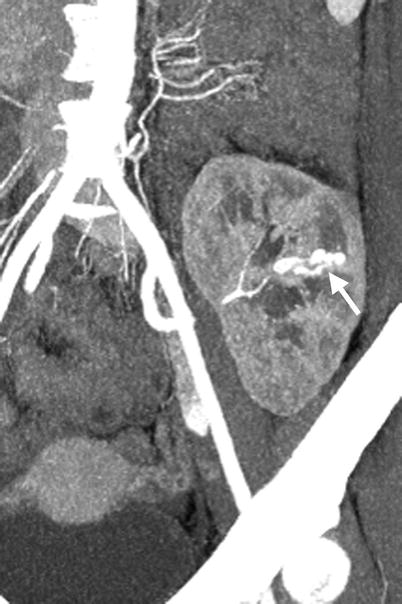
Fig. 11.13
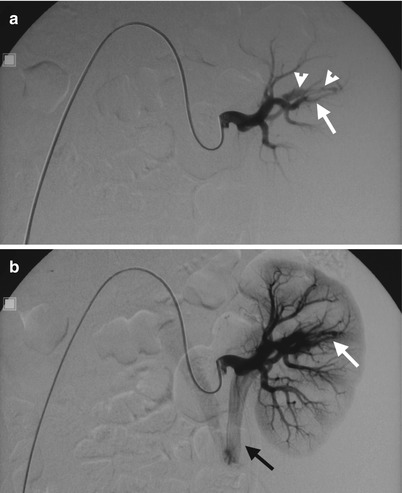
Fig. 11.14
Computed tomography angiography (CTA) and renal arteriography of allograft kidney show an arteriovenous fistula of allograft kidney after renal biopsy. CTA of allograft kidney (Fig. 11.13) shows an AVF (arrow) presenting as parallel enhancing vessels (double tram sign). Renal arteriography of the allograft kidney (Fig. 11.14a) shows opacification of early drainage vein (arrowheads) connecting to a renal arterial branch (arrow), suggestive of renal AVF. Renal arteriography of allograft kidney at 1 s later after Fig. 11.14a (Fig. 11.14b) shows early opacification of the main renal vein (black arrow) of the allograft kidney due to the presence of AVF (white arrow).
Key Diagnostic Features
Iatrogenic renal vascular injury could occur following percutaneous procedures such as percutaneous nephrostomy and renal biopsy or surgery including nephrectomy, partial nephrectomy, renal bisection, etc. [3, 4]. The iatrogenic renal vascular injury could present as renal arterial pseudoaneurysms, arteriovenous fistula (AVF), contrast medium extravasation, or even arteriocalyceal fistula [3, 4]. On renal angiography and computed tomography angiography, a renal arterial pseudoaneurysm shows a contrast medium pooling supplied by a renal artery which has delayed washout. Renal AVF could occur alone or in the presence of renal pseudoaneurysm. The presence of renal AVF on renal angiography is evidence by the presence of early drainage vein connecting the supplying renal arterial branch, and the ipsilateral main renal vein is also earlier opacified accordingly. Contrast extravasation on renal arteriography shows contrast medium flowing out the supplying artery and usually shows an irregularly shaped contrast pooling. Transcatheter arterial embolization could stop renal bleeding with successful clinical outcomes for patients with iatrogenic vascular injury [4].
11.7 Urine Leakage from Renal Collecting System After Renal Surgery
Case 12
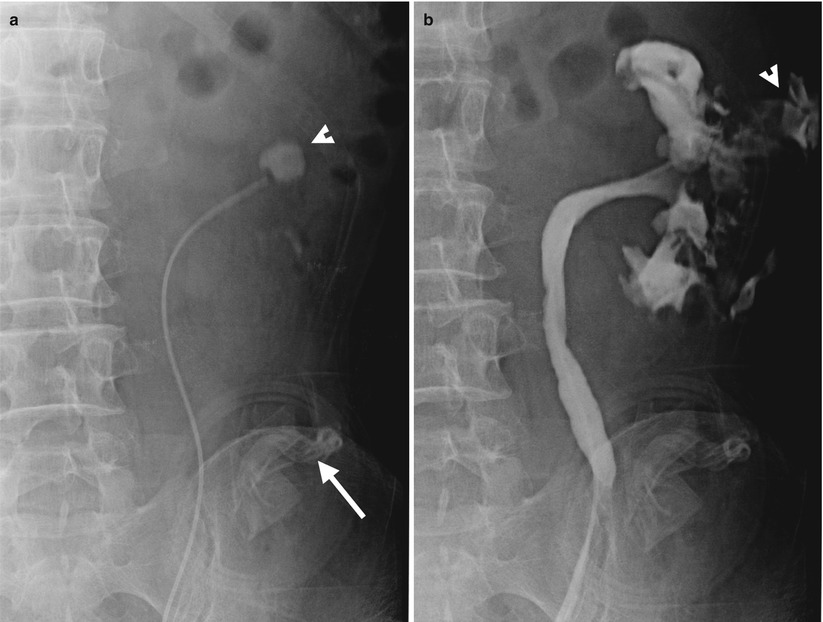
Fig. 11.15
Left retrograde pyelography (RP) shows imaging finding of contrast extravasation from the left renal collecting system in a patient after renal bisection for left renal staghorn stone. Baseline radiography of left RP before contrast medium injection (Fig. 11.15a) shows residual left renal stone (arrowhead) and drainage tubes (arrow) at the left abdomen. Left RP (Fig. 11.15b) shows contrast opacification of the left renal collecting system and left perirenal area (arrowhead), suggestive of urine extravasation from the left renal collecting system after surgery.
Case 13
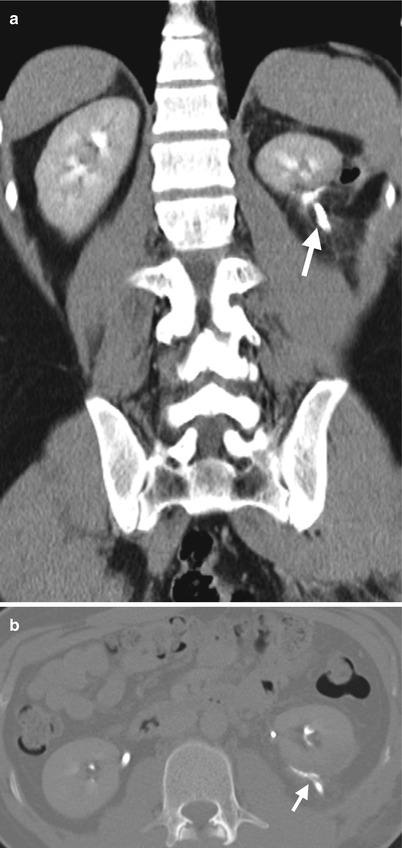
Fig. 11.16
Coronal CTU image (Fig. 11.16a) shows urine leakage from left renal lower pole to left perirenal area (arrow), evidenced by opacified contrast medium extravasation at excretory phase. Axial CTU image of wide window (Fig. 11.16b) shows conspicuous contrast leakage to left posterior perirenal area (arrow).
Key Diagnostic Features
The diagnosis of urine leakage from renal collecting system after renal surgery is usually straightforward. On retrograde pyelography (RP), the renal collecting system is normally opacified by contrast medium injection via the inserted ureteral stenting. If there is contrast opacification beyond the renal collecting system, urine leakage is suggested. On computed tomography (CT), excretory phase is usually necessary for confirmation urine leakage from the collecting system after contrast medium opacification. On the other hand, CT on arterial or nephrographic phase could be unable to make a diagnosis of urine leakage by the inability of confirmation of perinephric fluid being urine origin. Ureteral stenting and percutaneous nephrostomy have been commonly used for treatment of urine leakage from renal collecting system after surgery [5].
11.8 Iatrogenic Ureteral Trauma
Case 14
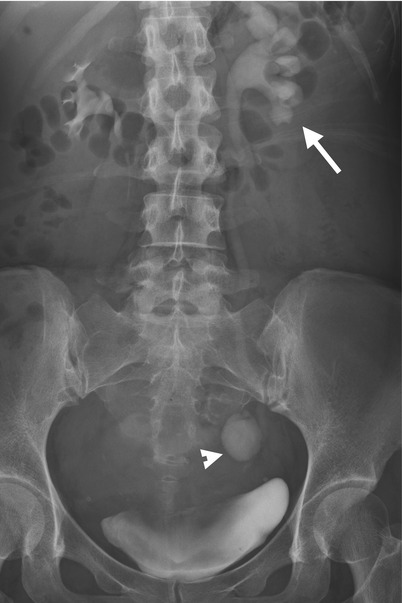
Fig. 11.17
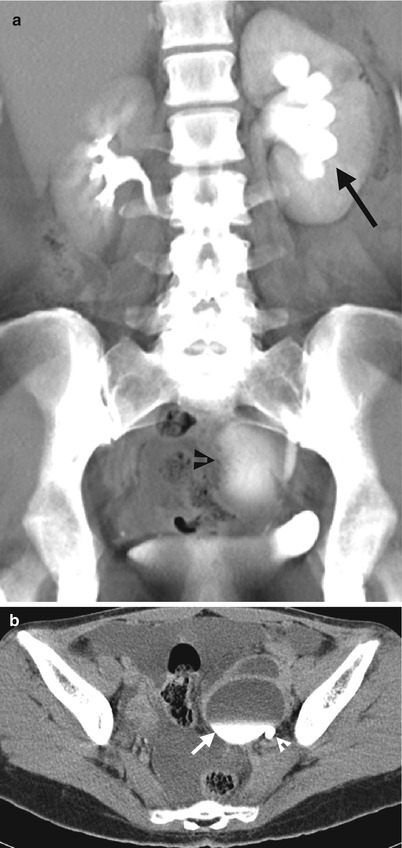
Fig. 11.18
Excretory urography (EU) and computed tomography urography (CTU) show imaging finding of urine leakage from the left distal ureter of a patient who has just undergone hysterectomy. Excretory urography at 30 min after contrast medium administration (Fig. 11.17) shows left hydronephrosis (arrow) and an oval-shaped contrast pooling area (arrowhead) of the left pelvic cavity. CTU of coronal reformatted image (Fig. 11.18a) shows similar left hydronephrosis (arrow) and contrast pooling (arrowhead) contiguous to the left distal ureter. Axial image of CTU (Fig. 11.18b) shows contrast leakage from the left distal ureter (arrowhead) which forms a urinoma (arrow) with contrast-fluid layering.
Case 15
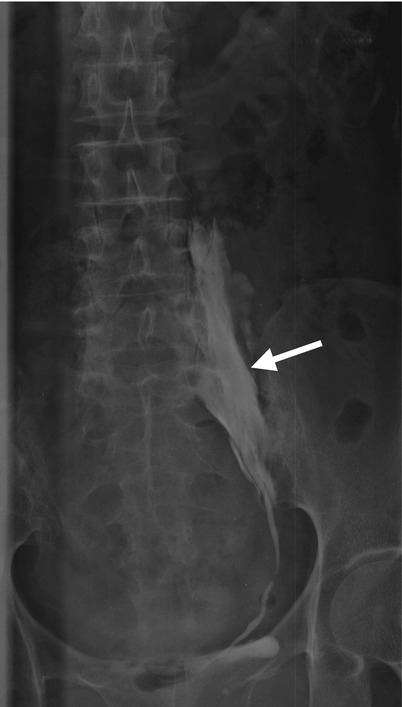
Fig. 11.19
Left retrograde pyelography (RP) (Fig. 11.19) shows contrast extravasation (arrow) along the left middle ureter with irregular profile due to left ureteral trauma during the procedure of ureteral stent insertion.
Key Diagnostic Features
Ureteral injury usually resulted from iatrogenic causes, most commonly by gynecological or colorectal surgery [6]. Occasionally, ureteral injury occurs during urological procedures [7]. Excretory urography (EU) and retrograde pyelography (RP) have been traditionally used to diagnose ureteral injury [7]. On RP and EU, contrast extravasation from the ureter has shown if there is ureteral wall defect in iatrogenic trauma. Computed tomography urography (CTU) could show imaging signs of ureteral rupture as well, which may include contrast extravasation from the ureter, hydronephrosis, urinoma formation, and hydroureter proximal to ureteral injury site. Transection or ligation of the ureter could be resulted from iatrogenic ureteral injury, too. In these cases, hydronephrosis with proximal hydroureter with complete nonvisualization of the ureter distal to the injury site is the main finding.
11.9 Stone of Allograft Ureter
Case 16
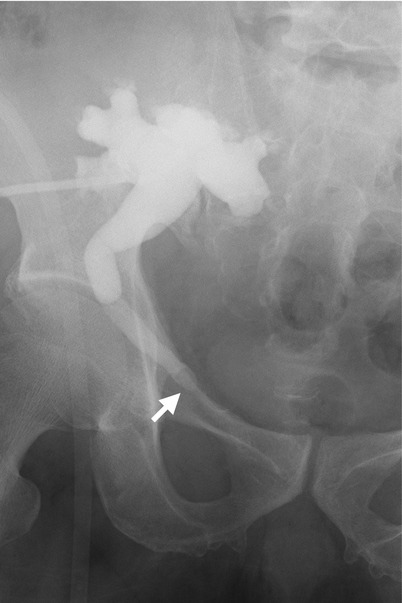
Fig. 11.20
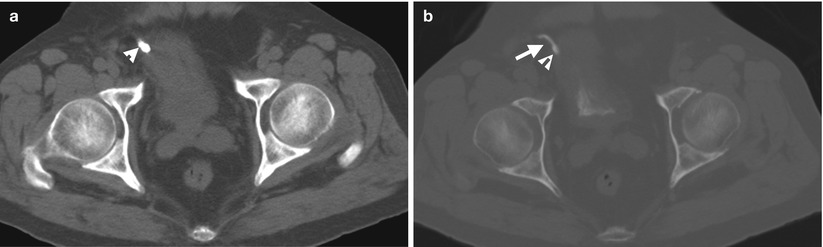
Fig. 11.21
Antegrade pyelography (AP) of the renal allograft (Fig. 11.20) shows oval-shaped filling defect (arrow) of the allograft ureter. Unenhanced (Fig. 11.21a) and enhanced excretory phase (Fig. 11.21b) computed tomography (CT show a high density stone (arrowheads), which is evidenced as in the opacified allograft ureter (arrow) on excretory phase of enhanced CT.
Key Diagnostic Features
Stone of allograft ureter is uncommon. On excretory urography (EU) and retrograde pyelography (RP), ureteral stone could appear as a filling defect of round or oval shape if it is detectable by sufficient size and radiopaque density. Unenhanced computed tomography (CT) is the most accurate for detecting ureteral stone of their high density. If there is uncertainty of the high density foci in the ureter or transplant ureter, enhanced CT of excretory phase is useful for localization and confirmation.
11.10 Bladder Rupture After Transurethral Resection of the Bladder Tumor (TURBT)
Case 17
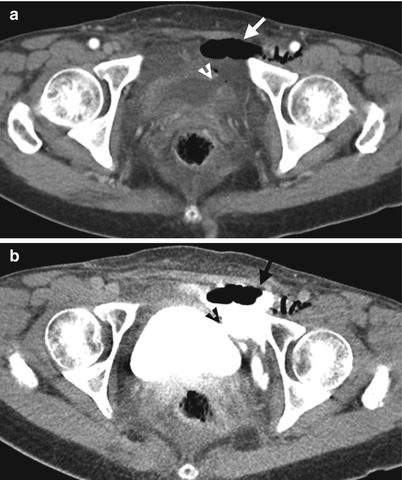
Fig. 11.22
Computed tomography (CT) shows imaging findings of extraperitoneal bladder rupture after TURBT. Unenhanced CT (Fig. 11.22a) shows a wall defect (arrowhead) of the left lateral wall of the urinary bladder. There is fluid and air collection (arrow) at the left anterior pelvic cavity near the urinary bladder. Enhanced CT of excretory phase (Fig. 11.22b) shows extravasation of opacified urine from the urinary bladder to the left anterior pelvic cavity (arrow) through the bladder wall defect (arrowhead).
Case 18
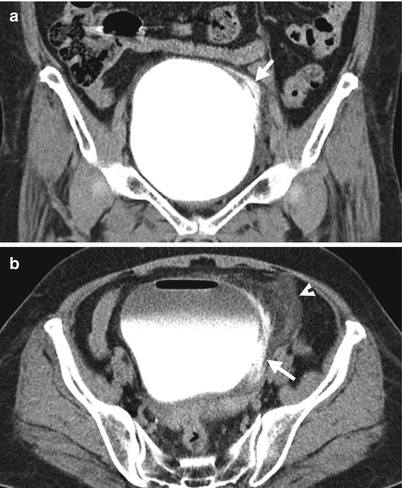
Fig. 11.23
Enhanced CT images of excretory phase on axial (Fig. 11.23a) and coronal (Fig. 11.23b) scans show contrast medium extravasation (arrows) from the left lateral wall of the urinary bladder to perivesical space. Note the dirty fat (arrowhead) in the left perivesical area close to the bladder rupture site.
Key Diagnostic Features
Bladder rupture after TURBT is an uncommon complication [8]. Most of them are extraperitoneal rupture of the urinary bladder [8]. Bladder rupture is diagnosed on cystography, computed tomography (CT) of excretory phase, or computed tomography cystography (CT cystography) by contrast extravasation from the urinary bladder. Sometimes, the wall defect responsible for bladder rupture could be seen on computed tomography. Among cystography, CT of excretory phase and CT cystography, CT cystography has the best accuracy for diagnosing bladder rupture by combination of pros of cystography as direct contrast medium injection into the urinary bladder cavity and CT as a cross-sectional imaging modality with better sensitivity to detect small contrast medium extravasation from the urinary bladder.
11.11 Irradiation Cystitis