Authors (year)
Target
Patients (Pts)
Staging
Equipment
PTV margin
Dose escalation
Outcomes
MTD
RD
LF
Failure Pattern
Whyte RI, et al. (Stanford Univ. & Cleveland clinic) (2003) [3]
1–5 cm in diameter, unresectable
15 pts
CT
Frameless stereotactic radiosurgery system (CyberKnife) Breath-holding technique (9 pts), Respiratory-tracking, robotic technique (14 pts)
N/A
15 Gy/single fraction
No grade 3–5 of RTOG complications
N/A
Response
N/A
8 pts (meta)
Spinal cord <8 Gy, brachial plexus <10 Gy, 2/3 of total lung <5 Gy, 50 % of heart <10 Gy, 50 % of esophagus <10 Gy, 50 % of liver <7.5 Gy
Complete, 2
Follow-up
Mean age 63
Partial, 15
1–26 mo
Stable, 4
(mean 7 mo)
Progressive, 2
Le QT, et al. (Stanford Univ.) (2006) [4]
Stage IA & IB NSCLC or metastatic tumor,
32 pts
CT & PET (29)
Linac-based, vacuum –set immobilization cushion,
2–5 mm
D95
Med f/u 18 mo
20 Gy ≤ <25 Gy/single fraction
≤20 Gy
1-year FFR
≤5 cm in diameter
T1, 6; T2, 14
Breath-holding technique or Synchrony respiratory tracking
system
15 Gy (n = 9)
25 Gy ≤
T2: 4/7,
NSCLC: 67 %,
Meta/rec, 12
20 Gy (n = 1)
Grade 2, 4 pts
met/rec: 1/3
met/rec: 25 %
Med age 72
25 Gy (n = 20)
Grade 3. 1 pt
25 Gy
30 Gy (n = 2)
Grade 5, 3 pts (central type)
T1: 0/5, T2: 0/6
met/rec: 4/9
30 Gy
T1: 0/1, T2: 1/1
McGarry et al.(Indiana Univ. group) [1]
Stage IA & IB,
47 pts,
CT & PET staging
Linac-based, body frame immobilization, abdominal compression for motion compensation
5 mm axial, 10 mm craniocaudal
D95: 3 × 8 Gy Cohorts of 3 patients received an additional 2.0 Gy /fr (total 6 Gy per increment at each dose escalation)
MTD
3 × 20 Gy (T1)
LF
T1
Tumors ≤7 cm, any location, medically inoperable
IA, 19; IIB, 28, med age 71(IA), 74(IIB)
3 × 22 Gy (T2)
3 × 22 Gy (T2)
4/19(T1), 6/28(T2)
1, regional alone
Not achieved (T1)
9 LF at doses 16 Gy /fr, med time 3–31 mo (mean, 15.7 mo).
4, distant
3, both
T2
2, regional alone
4, both
The Indiana University Dose Escalation Trial
At Indiana University a formal phase I dose escalation toxicity study was performed with 47 patients with medically inoperable non-small cell lung cancer (NSCLC) [1]. Three to five patients were treated within each dose cohort starting at 24 Gy in three fractions followed by successive dose escalations of 2 Gy per fraction (total increase per cohort, 6 Gy). Independent dose escalation trials were carried out in three separate patient groups: patients with T1 tumor, patients with T2 tumor less than 5 cm, and patients with T2 tumor 5–7 cm. In all cases, 95 % of the planning target volume (PTV) was covered by the 80 % prescription isodose volume. The PTV with setup uncertainty was designed from the gross tumor volume (GTV) by enlarging the volume 0.5 cm in the axial plane and 1.0 cm in the cranial-caudal plane in all directions. There was no limitation regarding the location of the tumor in the lung as both central and peripheral tumors were treated. Waiting periods occurred between dose cohorts to observe toxicity such as any grade 3 pulmonary, esophageal, cardiac or pericardial toxicity, or any grade 4 toxicity that was ascribed to the protocol treatment even after the acute period. A total of seven dose levels were tested. The maximal tolerated dose (MTD) was never reached for T1 tumors and T2 tumors less than 5 cm despite reaching 60–66 Gy in three fractions. For the largest tumors, dose was escalated all the way to 72 Gy in three fractions, which proved to be too toxic. Dose-limiting toxicity in that subset included pneumonia and pericardial effusion. Therefore, the MTD for tumors 5–7 cm in diameter was 66 Gy in three fractions, whereas the MTD for smaller tumors lies at an undetermined level beyond this dose.
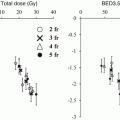
For all patients on the study, 9 local failures occurred at doses ≤16 Gy per fraction, whereas only 1 patient recurred at higher doses. A dose-response curve for local control using SBRT in lung cancer from these data is shown in Fig. 12.1. As the dose was increased more than 48 Gy, the local control rate at 17 months reached over 80 % [2]. From this study, a dose of 60–66 Gy in three fractions was determined to be reasonably safe for enrolled patients with medically inoperable NSCLC.
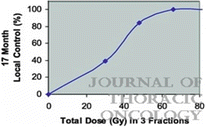

Fig. 12.1
Dose-response curve for local control after stereotactic body radiation therapy using three fractions in a prospective trial (Ref. [2])
Other Phase I Studies
Whyte et al. reported the preliminary results of a phase I study using a robotic frameless stereotactic radiosurgery system for early-stage lung cancer (n = 15) and metastatic lung tumors (n = 8) [3]. The enrolled patient were treated with a single dose of SBRT prescribing 15 Gy to the margin of the tumor at two institutions. With a mean follow-up of 7 months, toxicity was considered acceptable. Radiographic response was scored as complete in 2 patients, partial in 15, stable in 4, and progressive in 2.
In a Phase I dose-escalation study from Stanford University [4], 32 patients with inoperable T1–2 N0 NSCLC (n = 21) or solitary metastatic tumors (n = 11) received single fraction SBRT. Nine to 20 patients were treated per dose cohort starting at 15 Gy/fraction followed by dose escalation of 5–10 Gy to a maximal dose of 30 Gy/fraction. A minimal 3-month period was required between each dose level to monitor toxicity. At a median follow-up of 18 months, RT-related complications were noted for doses greater than 25 Gy and included four cases of grade 2–3 pneumonitis, one pleural effusion, and three possible treatment-related deaths. Especially, central tumor location and tumor volume were associated with a greater risk of severe to fatal toxicity.
Song et al. reported an experience in which they used a normal tissue complication probability (NTCP) formulation to guide dose escalation from 24 to 45 Gy in three fractions [5]. SBRT prescribed within the confines of NTCP-restricted dosing on this protocol resulted in no grade 3 or 4 radiation pneumonitis. However, late toxicity developed in two patients who received treatment to peri-hilar tumors, including one patient in whom bronchial stenosis developed with complete occlusion and lobar atelectasis 6 months after treatment.
12.2.1.2 European and Other Countries’ Experiences
European studies of SBRT [6–9] are shown in Table 12.2. In a retrospective study from Technical University in Germany [7], 68 patients with Stage I NSCLC received SBRT. Within the clinical protocol, SBRT was given starting at a total dose of 24 Gy in 4 fractions. It was planned to escalate total dose to 30 Gy in 3 fractions. Thereafter, the fractionation schedule and the single doses depended on lung function parameters, size and location of the target volume. For peripheral tumors 30–37.5 Gy in 10–12.5 Gy fractions was applied, and for central tumors 35 Gy in 7 Gy fractions was the standard schedule. Dose was prescribed to the 60 % isodose encompassing the PTV. Acute radiation pneumonitis occurred in 36 % of patients, while only one patient developed late grade 3 radiation pneumonitis (at 4 months) which progressed to fibrosis. One patient developed a grade 2 soft tissue fibrosis. With a mean follow-up of 17 months, no other grade >2 toxicity was observed. An actuarial local tumor control rate was 96 %, 88 % and 88 % after 1, 2 and 3 year follow-up, and an overall survival rate was 83 %, 71 %, and 51 % at 1, 2, and 3 years, respectively.
Table 12.2
European studies of SBRT for early-stage NSCLC
Toxicity | Outcomes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
Authors | Target | Patients (Pts) | Staging | Equipment | PTV margin | Dose | Acute | Late | LC, DFS | OS |
Nyman et al., Sahlgrenka Univ Hosp (Sweden) 2006 [6] | Stage IA & IB, tumors < 5 cm, noncentral | 45 pts (40 % IA), med age 74, med KPS 80 %, | CT only | Linac-based, body frame, abdominal compression for motion compensation | 5 mm axial, 10 mm craniocaudal | 45 Gy in 3 Fx (BED 112.5), Dmax approx 140 %, 100 % PTV coverage | 40 % Grade 1, 9 % Grade 2 (skin, cough, LRI) | 11 % (rib fx, Atx/fibrosis) | Med F/U 43 mo, LC 80 %, | 1/2/3/5 years = 80/71/55/30 % |
FFDM 80 % | Med OS 39 mo | |||||||||
20 % without histology | ||||||||||
Zimmermann et al., Technical Univ. (Munich) 2006 [7]
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||||||||||