Institute/trial [reference no.]
No. of patients (risk group)
Schedule
Free from PSA failure
Toxicities
Median follow-up
40 (Low)
33.5 Gy/5 fr
93 % at 5 years
1 acute Gr 3 toxicity; Late GU/GI
5 years
Gr2: 12.5 %/7.5 %, Gr3:2.5 %/0 %
41 (Low)
36.25 Gy/5 fr
100 %
GU Gr 2/3: 24 %/5 %
33 months
GI Gr 2/3: 15 %/0 %
67 (Low)
94 % at 4 years
GU Gr 1/2/3: 23 %/5 %/3 %
2.7 years
GI Gr 1/2/3: 12.5 %/2 %/0 %
Florida University [10]
102/10 (Low/Int.-High)
35 Gy/5 fr
98 %
GU Gr 3: 0
2 years
Rectal bleeding: 1 patient
50 (Low/Int./High: 45/5/0)
35 Gy/5 fr
Low/Int./High: 97 %/90.7 %/74.1 % at 5 years
Acute GU/GI Gr2: < 5 %
72 months
Late GU Gr2/3: 4 %/0 % 9 %
Late GI Gr2: 2 %
254 (Low/Int./High: 166/76/12)
36.25 Gy/5 fr
Acute GU/GI Gr2: < 5 %
60 months
Late GU Gr2/3: 9 %/2 %
Late GI Gr2: 5 %
Multi-institutional phase II [13]
211
40 Gy/5 fr
PSA failure: 1 pt
Acute GU/GI Gr 2: 20 %/8.5 %
18 months
Late GU/GI Gr2: 1 %/6 %
bladder necrosis (Gr3): 1 patient
Dose Escalation Study [14]
45 (15 for each dose group)
45 Gy/5 fr
100 %
GU/GI Gr2≤: 31 %/18 %, Gr3≤: 4 %/2 %
30 months
(Group of 50 Gy Acute GU/GI Gr2: 33 %/7 %, Gr3: 7 %/0 % Late GU Gr4: 7 %)
47.5 Gy/5 fr
18 months
50 Gy/5 fr
12 months
From Stanford University, King et al. reported the results of a single institutional phase II trial in which 41 patients with low-risk prostate cancer were treated by SBRT with a total dose of 36.25 Gy/5 fr. The PSA relapse-free survival rate was 100 % after a median follow-up period of 33 months [9]. The updated results showed that, in 67 patients with a median follow-up period of 2.7 years, the PSA relapse-free survival rate was 94 % at 4 years and the frequencies of long-term genitourinary (GU) and gastrointestinal (GI) toxicities were 3 % and 0 %, respectively [10]. Freidland et al. from Florida University also reported promising results for SBRT using 35 Gy/5fr [11]. Katz et al. treated more than 300 patients using schedules of both 35 Gy and 36.25 Gy, and reported long-term results with a median follow-up of 60 months. Comparison between the two schedules showed that the frequencies of late GU and GI toxicities tended to be higher in the 36.25-Gy group than in the 35-Gy group, although there was no significant difference [12, 13].
Meier et al. reported the results of a multi-institutional phase II trial using a schedule of 40 Gy/5 fr [14]. Boik et al. also reported on a multi-institutional phase I dose-escalation trial, which increased the total dose from 40 Gy, to 45 Gy, up to 50 Gy in 5 fractions [15]. In both trials, the conclusion was that high-dose SBRT over 40 Gy was safely implemented with good efficacy, but toxicity rates tended to be higher compared with moderate-dose SBRT with 35 Gy or 36.25 Gy in 5 fractions.
From the Stanford trial, King et al. compared toxicity rates between every-other-day (QOD) treatments and daily treatments (QD). QOD resulted in substantially less frequent Grade 1–2 GU toxicity (17 % vs. 56 %, p = 0.007) and less frequent Grade 1–2 GI toxicity (5 % vs. 44 %, p = 0.001) [9].
RTOG is now conducting a randomized phase II trial of SBRT (RTOG 0938), in which patients with favorable-risk prostate cancer are being randomized to receive either 36.25 Gy/5 fr (QOD) or 51.6 Gy/12 fr (QD), delivered by conventional linacs, CyberKnife, or protons [16].
15.1.3 Future Directions of SBRT for Prostate Cancer
A retrospective comparative analysis regarding toxicities between SBRT and IMRT for prostate cancer was recently published. Yu et al. analyzed more than 4,000 patients with prostate cancer treated by SBRT or IMRT, and reported that there appeared to be a greater rate of GU toxicity for patients undergoing SBRT compared with IMRT (15.6 % vs. 12.6 % at 6 months, p = 0.009; 43.9 % vs. 36.3 % at 2 years, p = 0.001) [17].
SBRT is a potentially beneficial treatment strategy for localized prostate cancer, but its clinical significance remains under investigation. Further prospective research including randomized trials is needed to determine the optimal schedule and to confirm efficacy and toxicity.
15.2 Spine SBRT
15.2.1 Introduction
Spine stereotactic body radiotherapy (SBRT) is an emerging treatment for patients with spinal metastases that is rapidly being adopted without level 1 evidence, particularly in North America [18]. The aim of this review is to update information concerning spine SBRT in relation to its efficacy and associated complications.
15.2.2 Efficacy
To date, results have been reported from two phase 2 trials and several prospective and retrospective studies.
Xin et al. reported the first clinical trial (phase 1–2 trial) of 149 patients with 166 lesions [19]. In that trial, significant reductions were observed in the severity of patient-reported pain between baseline and both 4 weeks post-treatment (mean, 3.4 [Standard Deviation (SD) 2.9] at baseline, 2.1 [2.4] at 4 weeks on the BPI pain at its worst item [0–10 scale]; effect size 0.47, p = 0.00076) and 6 months post-treatment (mean of 3.4 [SD 2.9] at baseline, 1.7 [2.4] at 6 months; effect size 0.64, p < 0.0001). The proportion of patients reporting no spine pain on the BPI increased significantly between baseline and 4 weeks post-treatment, from 49 of 149 (26 %) to 43 of 109 (39 %) (p = 0.038). This improvement continued throughout the study, with 53 of 120 patients (44 %) reporting no pain at 3 months (p = 0.004), and 55 of 102 patients (54 %) reporting no pain at 6 months (p < 0.0001) (Fig. 15.1).
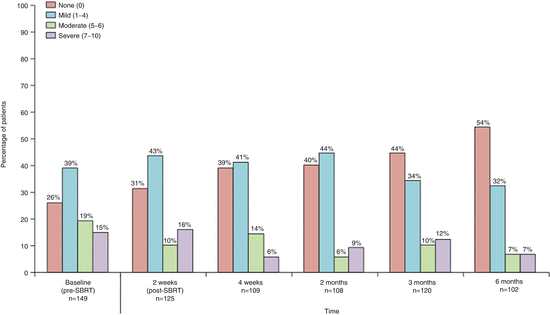

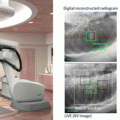
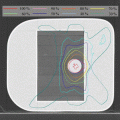
Fig. 15.1
SBRT plan for prostate cancer by CyberKnife (35 Gy/5 fr)
Peter et al. reported one of the biggest prospective evaluations, of 500 cases [20]. In that report, long-term pain control was achieved in 290 of the 336 cases (86 %), and long-term radiographic control was observed in 88 % cases. Pain and radiographic outcomes for the common histopathologies are shown in the Table 15.2.
Table 15.2
Summary of pain and radiographic outcome for the four most common histopathologies (n = 294)
Long-term pain improvement | |
All patients | 86 % |
Renal cell | 94 % |
Breast | 96 % |
Lung | 93 % |
Melanoma | 96 % |
Long-term radiographic control | |
All patients | 88 % |
Renal cell | 87 % |
Breast | 100 % |
Lung | 100 % |
Melanoma | 75 % |
15.2.3 Complications
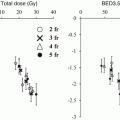
One of the most critical and dose-limiting organs at risk in safe SBRT practice is the spinal cord. Sahgal et al. reported a series of 9 cases of radiation-induced myelopathy (RM) that were compared with a cohort of 66 spine SBRT patients without RM [21]. In that report, dose-volume histograms (DVH) of the thecal sac were compared between the RM and no-RM cohorts.
Median doses to small volumes (smaller than 1 cc) differed significantly. These findings suggest that the small volume of the high-dose area in the thecal sac causes RM. They recommended limiting the maximum dose to the thecal sac to 12.4 Gy in 1 fraction, 17.0 Gy in 2 fractions, 20.3 Gy in 3 fractions, 23.0 Gy in 4 fractions or 25.3 Gy in 5 fractions to reduce the risk of RM to less than 5 %. (see Table 15.3).
Table 15.3
Comparison of median and mean nBED between the radiation myelopathy (RM) and no-RM cohorts
No-RM cohort (n = 66) (Gy2/2) | RM cohort (n = 9) (Gy2/2) | Mann–Whitney/t test (P value) | |
|---|---|---|---|
Median/mean Pmax volume nBED | 35.69/38.82 | 73.69/70.60 | .0003/.0006 |
Median/mean 0.1 cc nBED | 28.32/29.28 | 56.20/56.63 | .001/.006 |
Median/mean
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|