and John P. Deveikis2
(1)
Department of Surgery, Division of Neurosurgery, and Departments of Radiology and Neurology, University of Alabama, Birmingham, AL, USA
(2)
Bayfront Medical Center, St. Petersburg, FL, USA
Abstract
This chapter will discuss intracranial arterial stenosis and occlusion due to atherosclerosis and moyamoya syndrome. Approximately 8–10% of ischaemic strokes are attributable to intracranial atherosclerosis. In the USA, it is estimated that 40,000–60,000 new strokes per year are due to intracranial atherosclerosis.
This chapter will discuss intracranial arterial stenosis and occlusion due to atherosclerosis and moyamoya syndrome.
19.1 Atherosclerotic Intracranial Arterial Disease
19.1.1 Prevalence and Risk Factors
Approximately 8–10% of ischaemic strokes are attributable to intracranial atherosclerosis.1,2 In the USA, it is estimated that 40,000–60,000 new strokes per year are due to intracranial atherosclerosis.3
Distribution of symptomatic intracranial stenosis by location:
1)
Internal carotid – 20.3%
2)
MCA – 33.9%
3)
Vertebral artery – 19.6%
4)
Basilar artery – 20.3%
5)
Multiple arteries – 5.9%
19.1.1.1 Risk Factors
1)
Black, Asian, or Hispanic ethnicity5
a)
Black patients with TIA or stroke are more likely than white patients to have intracranial stenosis, whereas whites are more likely to have extracranial carotid atherosclerotic stenosis.1
i)
In a comparison of white and black patients with symptomatic posterior circulation disease, black patients had more lesions of the distal basilar artery, more high-grade lesions of intracranial branch vessels, and more symptomatic intracranial branch disease. Race was found to be the only factor increasing the risk of intracranial posterior circulation occlusive disease.6
b)
c)
A TCD study of healthy volunteers found a greater mismatch between cortical metabolic demand and cerebral blood flow in Asians compared to whites.9 This racial difference in neurovascular coupling may be a factor in the difference in rates of intracranial stenosis between the two groups.
2)
Hypertension is present in up to 75% of patients.10 Diabetes, coronary artery disease, cigarette smoking, and hypercholesterolemia, and peripheral arterial occlusive disease are also strongly associated.
3)
Patients without carotid bifurcation disease are more likely to demonstrate progression of intracranial stenosis compared with patients with it.11
4)
Cannabis use. Pot smoking has been linked to multifocal intracranial stenosis and ischaemic stroke in young people.12
5)
Metabolic syndrome is present in about 50% of patients with symptomatic intracranial atherosclerotic disease and is associated with a substantially higher risk of major vascular events.
a)
Metabolic syndrome is a cluster of interrelated risk factors that together increase an individual’s risk of cardiovascular disease.13 The syndrome consists of four main categories of metabolic abnormalities: atherogenic dyslipidemia (elevated triglycerides and decreased high-density lipoproteins), increased blood pressure, elevated plasma glucose, and a pro-thrombotic state. Some 24% of US adults have metabolic syndrome.14
19.1.1.1.1 Global Gem! Intracranial Stenosis
It is well established that intracranial arterial stenosis is more prevalent among Asian and African people compared to whites. Based on racial and ethnic patterns, intracranial stenosis may be the most important cause of ischaemic stroke in the world.
19.1.2 Aetiology of Symptoms
Ischaemic symptoms due to intracranial stenosis are believed to arise from the following:
19.1.3 Natural History
Intracranial stenoses are dynamic lesions that may demonstrate both progression and regression on serial imaging.11,20
1)
In a study of patients with intracranial stenosis undergoing repeat angiography at an average interval of 26.7 months, 40% of lesions were stable, 20% regressed, and 40% progressed.11
3)
Extracranial-intracranial (EC-IC) bypass surgery appears to promote progression of the lesion and occlusion of MCA in patients with nonoccluded MCA stenosis. 20
Asymptomatic intracranial stenosis is generally believed to be benign. In a series of 50 patients with asymptomatic MCA stenosis followed for a mean of 351 days, no patient had an ischaemic stroke in the corresponding territory.10
The best studies of the natural history of symptomatic stenosis have been from several prospective studies of medical therapy. Estimates of the overall annual ipsilateral stroke risk in patients with intracranial stenosis from prospective studies range from 2.3 to nearly 13%.4,7,21–24 The most definitive study so far is the prospective WASID trial, which found a first-year risk of ischaemic stroke in the pertinent vascular territory of 11–12%.4
The natural history of intracranial stenosis is somewhat dependent on the location of the lesion. Although a systematic review found no differences in recurrent ipsilateral stroke risk, overall mortality was found to be the lowest for patients with MCA stenosis.25
1)
Mean overall annual mortality:25
a)
MCA stenosis: 6.8%
b)
Vertebrobasilar stenosis: 11.6%
c)
Intracranial ICA stenosis: 12.4%
19.1.3.1 EC/IC Bypass Study
19.1.3.2 Warfarin Versus Aspirin for Symptomatic Intracranial Disease (WASID) Studies
The WASID studies evaluated two medical management strategies in the treatment of patients with symptomatic intracranial stenosis. Two separate studies were done. The first study was retrospective and suggested that warfarin is superior to aspirin.26 The second study was a prospective, multicenter, double-blinded randomized trial. Warfarin was associated with significantly higher rates of adverse events and did not provide a benefit over aspirin.4
19.1.3.2.1 wasid retrospective study
The retrospective study examined 151 patients with symptomatic intracranial atherosclerotic stenosis evaluated by angiography at seven centers between 1985 and 1991.26 Treatment consisted of either warfarin or aspirin and was determined at the treating physician’s discretion. The mean follow-up period was 14.7 months in the warfarin group and 19.3 months in the aspirin group. The annualized rate of stroke was 3.6% in the warfarin group and 10.4% in the aspirin group (p = 0.01), suggesting that warfarin is superior to aspirin in the treatment of patients with symptomatic intracranial stenosis. These findings lead to the organization of the prospective WASID trial.
19.1.3.2.2 wasid prospective trial
A total of 569 patients with TIA or stroke attributable to angiographically verified 50–99% stenosis of a major intracranial artery (Fig. 19.1) were randomized to receive warfarin (target INR, 2.0–3.0) or aspirin (1,300 mg per day).4 Enrollment was stopped prematurely (enrollment of 806 patients was originally planned) because of a significantly higher rate of haemorrhage in the warfarin group. The median time from qualifying event to randomization was 17 days, and the mean follow-up period was 1.8 years.
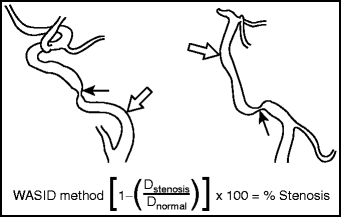

Fig. 19.1
WASID technique for measuring intracranial stenosis. The equation used for determining percent stenosis of a major intracranial artery in WASID.228 Dstenosis = the diameter of the artery at the site of the most severe degree of stenosis and Dnormal = the diameter of the proximal normal artery. Dnormal is selected according to the following criteria: (1) First choice (left): The diameter of the proximal part of the artery at its widest, nontortuous, normal segment is selected. Stenotic region (arrow); reference area (Dnormal) (open arrow). (2) Second choice (right): If the lesion is at the origin of the vessel, or if the proximal artery is diseased (e.g., proximal basilar artery stenosis or M1 segment origin stenosis), the diameter of the distal portion of the artery at its widest, parallel, nontortuous normal segment is used. Stenotic region (arrow); reference area (Dnormal) (open arrow). (3) Third choice: If the entire intracranial artery was diseased, the most distal, parallel, nontortuous normal segment of the feeding artery is measured.
1)
The primary end point of ischaemic stroke, brain haemorrhage, or death from vascular causes other than stroke:
a)
21.8% in the warfarin group
b)
22.1% in the aspirin group (p = 0.83)
2)
Rate of death:
a)
9.7% in the warfarin group
b)
4.3% in the aspirin group (p = 0.02)
3)
Major haemorrhage
a)
8.3% in the warfarin group
b)
3.2% in the aspirin group (p = 0.01)
4)
Myocardial infarction or sudden death:
a)
7.3% in the warfarin group
b)
2.9% in the aspirin group (p = 0.02)
5)
Rate of death from vascular causes:
a)
5.9% in the warfarin group
b)
3.2% in the aspirin group (p = 0.16)
6)
Rate of death from nonvascular causes
a)
3.8% in the warfarin group
b)
1.1% in the aspirin group (p = 0.05)
The risk of ischaemic stroke in the territory of the stenotic artery at 1 year in patients treated with aspirin was 12%, and in patients treated with warfarin the risk was 11% (p = 0.31). Because of the high adverse event rates for patients treated with warfarin, and lack of therapeutic benefit of warfarin over aspirin for prevention of ischaemic stroke caused by intracranial stenosis, the WASID investigators concluded that aspirin should be used in preference to warfarin for patients with intracranial arterial stenosis.
19.1.3.2.3 wasid prospective trial subgroup analyses
An analysis of selected subgroups of patients in the WASID trial found no advantage of warfarin over aspirin for preventing the primary end point of ischaemic stroke, brain haemorrhage, or vascular death.27 A statistically significant benefit was associated with warfarin in patients with symptomatic basilar artery stenosis in terms of the primary end point (ischaemic stroke, brain haemorrhage, or death from vascular causes other than stroke). However, the sample size and wide confidence intervals of this finding diminished the credibility of this finding. Furthermore, there was no difference in the rates of ischaemic stroke in the territory of the symptomatic basilar artery between treatment group, and no benefit was found in patients with symptomatic intracranial vertebral artery stenosis. The WASID investigators concluded that warfarin demonstrated no convincing benefit in patients with basilar artery stenosis.
19.1.3.2.4 wasid predictors of ischaemic stroke in the territory of a symptomatic intracranial stenosis
The majority of strokes (73%) in WASID patients were in the territory of the stenotic artery.28 The risk of stroke in the territory of the stenotic artery was greatest in patients with the following characteristics:
1)
Severe (≥70%) stenosis (p = 0.0025).
2)
Patients enrolled early (≤17 days) (p = 0.028).
3)
There was a statistical trend toward an increased risk for women (p = 0.051)
4)
Location of stenosis, type of qualifying event, and prior use of antithrombotic medications were not associated with increased risk.
19.1.3.2.5 gesica study
The GESICA Study (Groupe d’Etude des Sténoses Intra-Crâniennes Athéromateuses symptomatiques) was a prospective, multicenter nonrandomized study in France.24 A total of 102 patients with symptoms attributable to intracranial stenosis ≥50% indicated by either angiography or ultrasonography were enrolled. Optimal medical therapy was left to the discretion of the local investigators. The mean follow-up period was 23.4 months.
1)
The annualized risk of a cerebrovascular event (TIA or stroke) in the territory of the affected artery was 19.2%.
a)
Annualized risk of TIA: 12.6%.
b)
Annualized risk of stroke: 7.0%
19.1.4 Medical Treatment of Symptomatic Intracranial Stenosis
Medical management of symptomatic intracranial stenosis consists of antiplatelet therapy, strategies to treat hyperlipidaemia, and aggressive control of medical risk factors, such as diabetes, hypertension, and cigarette smoking. Medical therapy of patients with cerebral ischaemia is discussed in detail in Chap. 17: Acute Ischaemic Stroke. The authors of this handbook favor the following regimen for patients with symptomatic intracranial stenosis:
1)
AggrenoxTM (Boehinger Ingelheim Pharmaceuticals, Inc.)
a)
Aspirin (25 mg) plus extended-release dipyridamole (200 mg) PO BID has been found in a randomized trial to reduce risk of recurrent stroke.29
2)
Atorvastatin (Lipitor®, Pfizer, Inc.)
a)
High-dose atorvastatin (80-mg PO QD) was found in a randomized trial to reduce the risk of recurrent stroke.30
b)
Note: Myalgia is a common side effect; myopathy and rhabdomyolysis occur in <0.01% of patients. Blood work including serum creatinine, creatine kinase (CK), and liver function tests should be done prior to starting the drug and at 3 months on the drug.
3)
Antihypertensive agents, as needed.
4)
Tight control of serum glucose, for diabetic patients.
5)
Smoking cessation.
19.1.5 Intracranial Angioplasty and Stenting
Intracranial angioplasty with or without stenting is beginning to emerge as an acceptable treatment in very selected patients. However, the efficacy of the technique is difficult to assess from the existing literature due to (1) rapidly evolving technology; (2) a wide variety of techniques reported in the literature; and (3) a paucity of prospective data, and the complete absence of randomized trial data. Most single-center series have reported on angioplasty alone,31–35 or angioplasty and stenting with balloon-mounted coronary stents.34,36 Angioplasty without stenting is associated with a significant risk of restenosis,31 which has lead to interest in angioplasty with stenting. Balloon-mounted coronary stents, however, are limited by the low flexibility of coronary stent system, the high inflation pressures needed to deploy balloon-mounted stents in fragile intracranial vessels, and the risk of shearing the stent from the balloon while navigating to the target lesion.37 The best studies of intracranial angioplasty and stenting are the SSYLVIA 38 and Wingpan 37 studies, both of which were prospective, nonrandomized studies using devices specifically designed for the treatment of intracranial stenosis.
A Cochrane systematic review of 79 publications of intracranial angioplasty with or without stenting found the following:39
1)
Overall perioperative stroke rate of 7.9%
2)
Perioperative death rate of 3.4%
3)
Perioperative rate of stroke or death of 9.5%
19.1.5.1 Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA)
The SSYLVIA trial was a multicenter, nonrandomized, prospective feasibility study of the Neurolink® intracranial stent system (a product of the Guidant Corporation, which is now part of Boston Scientific) for treatment of vertebral or intracranial artery stenosis.38 The Neurolink stent is a balloon-mounted device. A total of 43 patients with symptomatic intracranial stenosis and 18 patients with extracranial vertebral artery stenosis were enrolled.
1)
Successful stent placement was achieved in 95% of cases.
2)
Thirty-day periprocedural stroke rate: 6.6%. No deaths occurred. Two strokes occurred during the procedure.
3)
At 6-month angiographic follow-up, restenosis of >50% occurred in 32.4% of intracranial vessels and 42.9% of extracranial vertebral arteries.
a)
39% of the recurrent stenoses were symptomatic.
4)
Strokes in the distribution of the target lesion occurring after 30 days but by 12 months were seen in 7.3% of patients.
Based upon this study, the FDA granted a humanitarian device exemption to treat patients with significant intracranial and extracranial atherosclerotic disease by balloon angioplasty and stent placement. The manufacturer is not currently marketing the Neurolink device, in favor of the Wingspan® system.
19.1.5.2 Wingspan Study
The WingspanTM Stent System with GatewayTM PTA Balloon Catheter (Stryker, Fremont, CA) was designed for the treatment of intracranial atherosclerotic stenosis. Prestent dilation of the lesion is done with the angioplasty balloon, and the stent, a self-expanding nitinol device, is then deployed. The device received an FDA humanitarian device exemption in August 2005 (http://www.fda.gov/cdrh/mda/docs/h050001.html). The Wingspan Study was a prospective, multicenter nonrandomized study of the devices in medically refractory patients with recurrent symptoms attributable to in intracranial stenosis ≥50% in a vessel 2.5–4.5 mm in diameter.37 A total of 45 patients were enrolled. The mean initial degree of stenosis was 77.9%, and the mean lesion length was 7.2 mm.
1)
The stent was successfully deployed in 97.8% of cases.
a)
Procedural adverse events were reported in 27% of patients but none resulted in permanent sequelae.
2)
The degree of stenosis was reduced from a baseline of 74.9–31.9% after stenting.
3)
The 30-day composite stroke/death rate was 4.5%.
4)
Clinical follow-up at 6 months:
a)
Ipsilateral stroke/death rate: 7.0%
b)
Incidence of all strokes: 9.7%
c)
All-cause mortality: 2.3%
5)
Angiographic follow-up at 6 months:
a)
Mean degree of stenosis: 28%.
b)
Three patients (6.8%) showed restenosis >50%; all were asymptomatic.
In contrast to SSYLVIA, which reported a rate of restenosis >50% of 32.4% at 6 months, the mean degree of stenosis at 6 months in the Wingspan Study was not significantly different from the degree of stenosis immediately after the procedure.
19.1.5.3 Other Notable Studies
19.1.5.3.1 mori 1998
Mori et al. reported on angioplasty without stenting in 42 patients with intracranial stenosis >70% stenosis.32,40 The risk of recurrent stenosis was strongly associated with lesion length and complexity:
Lesion length and geometry | Rate of restenosis at 3 months | |
|---|---|---|
Type A | ≤5 mm, concentric or moderately eccentric | 0 |
Type B | 5–10 mm, extremely eccentric or totally occluded | 30.8% |
Type C | >10 mm, >90% angulation, or totally occluded | 66.7% |
19.1.5.3.2 marks 2006
Report of 120 patients with intracranial stenosis ≥50% who were treated with angioplasty without stenting.35 A total of 116 patients were available for a mean follow-up time of 42.3 months.
(a)
Degree of stenosis was reduced by angioplasty from a mean of 82.2–36.0%.
(b)
Combined 30-day periprocedural stroke and death rate was 5.8%.
(c)
Annual postprocedure stroke rate was 3.2% in the territory of treatment and annual overall stroke rate was 4.4%.
19.1.5.3.3 systematic review comparing angioplasty alone to angioplasty and stenting
A systematic review of 69 studies of angioplasty alone and 36 studies of angioplasty with stent placement:41
1)
Technical success
a)
Angioplasty alone: 79.8%
b)
Angioplasty and stenting: 95% (P < 0.0001)
2)
Pooled incidence of 1-year stroke and/or death
a)
Angioplasty alone: 19.7%
b)
Angioplasty and stenting: 14.2% (P = 0.009)
3)
Pooled restenosis rate
a)
Angioplasty alone: 14.2%
b)
Angioplasty and stenting: 11.1% (P = 0.04)
The authors concluded that angioplasty and stenting may be superior to angioplasty alone in this setting.
19.1.5.4 Position Statement on Intracranial Angioplasty and Stenting for Cerebral Atherosclerosis by the ASITN, SIR, and ASNR3
1)
For symptomatic patients with >50% intracranial stenosis, who have failed medical therapy, balloon angioplasty with or without stenting should be considered.
2)
Patients who have an asymptomatic intracranial arterial stenosis should first be counseled regarding optimizing medical therapy. There is insufficient evidence to make definitive recommendations regarding endovascular therapy in asymptomatic patients with severe intracranial atherosclerosis. They should be counseled regarding the nature and extent of their disease, monitored for new neurological symptoms, and have periodic noninvasive imaging at regular intervals of 6–12 months (MRA or CTA) initially, and then by cerebral angiography if warranted. At a minimum, optimal prophylactic medical therapy should be instituted, which might include antiplatelet and/or statin therapy.
3)
Continued evaluation and improvements in both pharmacological and catheter-based therapies are needed to reduce the stroke burden from intracranial atherosclerosis.
Note: ASITN American Society of Interventional and Therapeutic Neuroradiology, SIR Society of Interventional Radiology, ASNR American Society of Neuroradiology
19.1.5.5 Stenting Versus Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS)
Four hundred and fifty one patients with symptomatic 70–99% stenosis of a major intracranial artery were randomized to either aggressive medical management alone (n = 227) or aggressive medical management plus Wingspan angioplasty and stenting (n = 224).42 Enrollment was stopped prematurely (764 patients were planned) because an interim analysis showed significantly better results for the medical management-only group. Mean follow-up was 11.9 months.
1)
One year primary end point rate (stroke or death within 30 days after enrollment or stenting, or stroke in the territory of the qualifying artery beyond 30 days):
a)
20.0% in the stenting group
b)
12.2% in the medical management-only group (p = 0.009)
2)
30-day rate of stroke or death:
a)
14.7% in the stenting group
b)
5.8% in the medical management-only group (p = 0.002)
3)
30-day rate of symptomatic intracranial haemorrhage:
a)
4.5% in the stenting group
b)
0% in the medical management-only group
4)
Medical management was identical in both groups and consisted of aspirin 325 mg per day and clopidogrel 75 mg per day for 90 days after enrollment; blood pressure management (target systolic blood pressure <140 mmHg, <130 mmHg in patients with diabetes); hyperlipidemia (target LDL <70 mg/dL); and help with compliance and management of diet, smoking, weight reduction, and exercise with a lifestyle modification program. Medications provided by the study include aspirin, clopidogrel, one drug from each major class of antihypertensive agents, and rosuvastatin.
5)
SAMMPRIS controversies
a)
The initial results were a surprise for many people. Like any major study showing unexpected results, there has been a fair bit of controversy over the study findings and their applicability to clinical practice. This debate will likely rage for years. Here are some of the arguments made in the debate about the SAMMPRIS results:
i)
The aggressive medical management of patients in SAMMPRIS does not resemble real-world medical management of patients with intracranial stenosis.
ii)
The adverse event rate in the stenting arm was unacceptably high.
iii)
The results of SAMMPRIS may lend support to the use of angioplasty alone, rather than angioplasty and stenting, for intracranial stenosis.
(1)
Although angioplasty alone may be safer than angioplasty and stenting, a study comparing angioplasty alone to medical management has not yet been published. A systematic review found angioplasty and stenting to be superior to angioplasty alone.41
iv)
The results of SAMMPRIS show that the FDA Humanitarian Device Exemption process needs to be reviewed.
19.1.5.6 Phase III Study of PHAROSTM VitesseTM Neurovascular Stent System Compared to Best Medical Therapy for the Treatment of Ischaemic Disease (VISSIT)
Industry-sponsored randomized trial of intracranial angioplasty and stenting using the PHAROSTM VitesseTM (Codman Neurovascular, San Jose, CA) balloon-expandable stent versus medical therapy.43 Entrance criteria are similar to SAMMPRIS. This study was discontinued in early 2012.
19.2 Moyamoya Disease and Moyamoya Syndrome
Moyamoya disease (aka spontaneous occlusion of the circle of Willis) is a nonatherosclerotic progressive steno-occlusive arteriopathy that most frequently affects the intracranial ICAs and proximal segments of the MCAs and ACAs. It may rarely also involve the posterior circulation. Spontaneous occlusion of the major intracranial arteries is typically accompanied by the appearance of a tuft of fine collateral vessels at the base of the brain. Moyamoya is a Japanese word meaning puff of smoke, or ambiguous, which are descriptions not only of the tuft of collaterals but also the obscure aetiology of the syndrome, which remains unelucidated.44 The term moyamoya disease is reserved for those cases in which the intracranial vascular changes are primary and truly idiopathic, whereas moyamoya syndrome (aka secondary moyamoya, moyamoya phenomenon, syndromic moyamoya, quasi-moyamoya, or moyamoya-like vascular changes) is used with the intracranial vascular changes that occur in association with another condition, such as cranial radiation or neurofibromatosis type 1.45
19.2.1 Epidemiology
Ethnicity plays a major role. Japan and South Korea appear to have the highest concentrations of patients with moyamoya disease.
1)
Japan:
b)
Prevalence rate of 3.16 and annual incidence of 0.35 per 100,000.
c)
Male to female ratio: 1:1.8.
d)
Peak ages are 10–14 years and 40s.
i)
Age at onset is <10 years in 47.8% of the patients; some develop the disease at the age of 25–49 years.
ii)
Bony carotid canal hypoplasia has been found in Japanese patients with adult-onset moyamoya disease, suggesting that the pathological process begins early in life in all patients.48
2)
South Korea:
a)
Female predominance and bimodal age distribution pattern are similar to those seen in Japanese patients. The incidence of adult moyamoya disease in South Korea is 20% higher than that in Japanese patients, and the incidence of familial moyamoya syndrome is only 1.8%.49
3)
China:
a)
Data on moyamoya in China is very limited. Two reports indicate the following:
b)
c)
One report has attributed the majority of cases (53%) in China to leptospiral arteritis, and found that 81.4% of patients with moyamoya syndrome have positive leptospiral test results.50
4)
North America:
a)
The overall incidence of moyamoya syndrome in California and Washington is 0.086 per 100,000.52
i)
Male to female ratio: 1:2.15
b)
Incidence per 100,000 is highest among Asian Americans and lowest among Hispanics:
i)
Asian Americans: 0.28
ii)
African Americans 0.13
iii)
Whites: 0.06
iv)
Hispanics: 0.03
c)
Median age of onset is as follows:
i)
Asian Americans: 36
ii)
African Americans: 18
iii)
Whites: 32
iv)
Hispanics: 21
d)
Sickle cell disease likely accounts for the relatively high incidence and low age of onset among African Americans; when patients with sickle cell disease were excluded, the incidence among African Americans was similar to that in whites.
e)
The prevalence of moyamoya disease in Hawaii appears to be higher than in the rest of the USA.53
5)
Europe:
a)
A survey of European centers lead to an estimate of the incidence of moyamoya to be about 1/10 the incidence in Japan.54
b)
A slightly higher incidence in Eastern Europe has been hypothesized to be due to the Mongolian invasion of Europe, which may have spread the genetic predisposition for moyamoya disease.55
19.2.2 Pathophysiology
The primary lesion in moyamoya disease is progressive fibrocellular thickening of the intima.56–58 The intima acquires an onion-like appearance, consisting of fibrocellular materials, but without lipids or calcification as is seen in atherosclerosis.59 The internal elastic lamina is also abnormal, becoming infolded, tortuous, redundant, and fragmented.60 The media is thinned, with a diminished number of smooth muscle cells.58 No inflammatory changes are seen. Intimal thickening has also been found in the superficial temporal arteries of patients with moyamoya.61 Histological analysis has shown thrombotic lesions in the affected intracranial vessels in 54% of cases.62
The secondary lesions in moyamoya syndrome are dilated, tortuous thalamostriate and lenticulostriate arteries at the base of the brain. These vessels do not appear to be normal collateral vessels that would be expected to develop in response to chronic occlusion of major arteries. They exhibit thinned and fragmented internal elastic laminae, medial fibrosis, microaneurysms, and areas of rupture.56 Stenosis of these vessels, due to fibrous intimal thickening and thrombosis, is seen in 50% of cases.56
The aetiology of moyamoya disease is unknown. Several mechanisms have been implicated:
1)
Primary defect in smooth muscle cells.
a)
Deoxyribonucleic acid synthesis experiments involving cultured smooth muscle cells from moyamoya patients indicate that the cells are less responsive to their normal mitogens. This suggests that there be a derangement in the vessel wall repair mechanism that leads to long-term proliferation of cells and progressive occlusion of the vessel lumen.63
2)
Role of angiogenic factors.
a)
Basic fibroblast growth factor (bFGF). Elevated levels of bFGF have been found in the CSF,64,65 superficial temporal artery,66 and in the affected ICA.67 of patients with moyamoya disease. Basic FGF is a potent angiogenic factor. It is not clear whether elevation of bFGF is a primary event in the pathogenesis of moyamoya, or an epiphenomenon in response to chronic ischaemia. Interestingly, elevated CSF bFGF correlates with the degree of angiogenesis seen with indirect revascularization procedures in patients with moyamoya disease.65,68
b)
Elevated expression of transforming growth factor beta 1 has been found in cultured smooth muscle cells and in serum from patients with moyamoya disease.69
c)
Hepatocyte growth factor, an angiogenic factor, is elevated in CSF and intracranial arteries in patients with moyamoya disease.70
3)
Alteration in metaloproteinase gene expression.
a)
An association analysis of tissue inhibitor of metalloproteinase 2 in 17q25 showed that a polymorphism in the promoter region was markedly associated with familial moyamoya disease.71
4)
Excessive prostaglandin synthesis.
a)
Cultured smooth muscle cells from moyamoya patients produce excess amounts of prostaglandin E(2) in response to stimulation with interleukin-1 beta.72 This may increase vascular permeability and exposure of the vessels to blood constituents, leading to intimal thickening.
5)
Epstein-Barr virus (EBV) infection.
a)
Antibody titers of EBV are significantly elevated in patients with moyamoya disease,73 raising the possibility that EBV infection may be involved.
19.2.3 Diagnosis of Moyamoya Disease
The Ministry of Health and Welfare in Japan has established guidelines for the diagnosis of moyamoya disease.74 The diagnosis may be made by catheter angiography or MRI and MRA. Cases are defined as either definite or probable, depending on which criteria are satisfied.
19.2.3.1 Diagnostic Criteria
1)
Cerebral angiography should demonstrate the following findings:
a)
Stenosis or occlusion at the terminal portion of the ICA and/or the proximal portion of the ACA and/or MCA.
b)
Abnormal vascular networks in the vicinity of the occlusive or stenotic lesions.
c)
These findings should be present bilaterally.
2)
When MRI and MRA clearly demonstrate all of the findings listed later, catheter angiography is not mandatory.
a)
Stenosis or occlusion at the terminal portion of the ICA and at the proximal portion of the ACA and MCA.
b)
An abnormal vascular network in the basal ganglia on MRA. An abnormal vascular network can be diagnosed on MRI when >2 apparent flow voids are seen in one side of the basal ganglia.
c)
(a) and (b) are seen bilaterally.
3)
Exclude other causes. Because the aetiology of the disease is unknown, associated cerebrovascular diseases or conditions should be excluded. These include, but are not limited to the following:
a)
Arteriosclerosis
b)
Autoimmune disease
c)
Meningitis
d)
Brain neoplasm
e)
Down syndrome
f)
Neurofibromatosis type 1
g)
Head trauma
h)
Cranial irradiation
4)
Pathological findings that can be helpful in the diagnosis are as follows:
a)
Intimal thickening that causes stenosis or occlusion of the lumen is observed in and around the terminal portion of the internal carotid artery. Lipids deposition is occasionally seen in the proliferating intima.
b)
Arteries of the circle of Willis often show stenosis or occlusion associated with fibrocellular thickening of the intima, waving of the internal elastic lamina, and attenuation of the media.
c)
Numerous small arteries (perforating and anastomotic branches) are observed around the circle of Willis.
d)
Reticular conglomerates of small vessels are often seen in the pia mater.
19.2.3.2 Diagnosis
1)
Definite case: Either all criteria in (1) or all criteria in (2) and (3) are fulfilled. In children, however, a case that fulfills (1), (a) and (b) or (2), (a) and (b) on one side and demonstrates narrowing at the terminal portion of the internal carotid artery on the opposite side is also considered to be a definite case.
2)
Probable case: Either: (1), (a) and (b) or (2), (a) and (b) and (3) are fulfilled (i.e., unilateral involvement).
19.2.4 Evaluation
19.2.4.1 CT
Significant abnormal findings on CT are present in 92% of cases.75 These include cortical atrophy, ventricular dilatation, and irregular, multiple or bilateral lucent areas in the cortex, white matter, and central gray matter.
19.2.4.2 Angiography
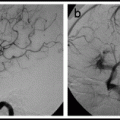
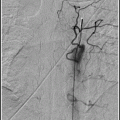
Although a diagnosis of moyamoya disease may be made with MRI and MRA alone,74 the authors of this handbook recommend catheter angiography in most young patients and all adults suspected of having moyamoya disease or moyamoya syndrome. Angiography in children with moyamoya disease is not associated with a higher rate of complications compared with angiography in children without moyamoya.76 Angiography is the best imaging technique to distinguish high-grade stenosis from occlusion, identify intracranial aneurysms, and assess collateral circulation. Importantly, angiography is critical for planning surgical procedures; for instance, angiography can best demonstrate transdural collateral vessels that should be preserved during revascularization procedures.
1)
Angiographic staging of moyamoya disease is summarized in Table 19.1. Stage 4 is the most common stage at presentation. Angiographic stage is usually more advanced in more elderly patients.77
Table 19.1
Angiographic stages of moyamoya disease
Stage | Angiographic findings |
|---|---|
1 | Stenosis of the supraclinoid ICA. Usually bilateral. No other abnormalities. |
2 | Initiation of moyamoya vessels at the base of brain. |
3 | Intensification of moyamoya vessels. Increased stenosis or occlusion of the ICA, MCA, or ACA. |
4 | Complete occlusion of the ICA with some reduction in moyamoya vessels. First appearance of collaterals from ECA branches. Most common stage at presentation. |
5 | Further reduction of moyamoya vessels with increased collateralization from ECA. |
6 | Complete absence of major intracranial arteries and moyamoya vessels. Uncommon. |
2)
Stenosis or occlusion of the intracranial ICA and/or the A1 and M1 segments are seen in all cases.
4)
5)
Abnormal moyamoya vessels may be seen in several locations.
a)
Basal ganglia – branches of the circle of Willis
c)
Cerebral hemispheres (i.e., vault moyamoya) – branches of the anterior falx, middle meningeal, occipital, tentorial, and superficial temporal arteries82
6)
The extensive development of basal moyamoya is a sign of severe haemodynamic impairment in adult patients with ischaemic moyamoya disease.84
8)
Intracranial aneurysms are present in about 10% of cases.86–89 They are attributable to abnormal haemodynamic patterns, or for those that arise from moyamoya vessels, abnormal vessel architecture. Aneurysms are found in three locations:90
a)
Circle of Willis, mostly in the posterior circulation: 60% of cases.
b)
Peripheral cerebral arteries, such as the anterior or posterior choroidal arteries: 20%.
c)
Abnormal moyamoya vessels: 20%.
9)
Persistent primitive arteries have been observed in both moyamoya disease and moyamoya syndrome at higher frequencies that in the general population.91
19.2.4.3 MRI
1)
2)
3)
In a series of 30 patients with moyamoya disease, all showed clear evidence on MRI of bilateral stenosis or occlusion of the supraclinoid ICA and proximal ACAs and MCAs.93 Parenchymal lesions were apparent on MRI in 80% of cases. White matter infarctions are the most frequent parenchymal lesions and are seen in 73% of cases.
19.2.4.4 Cerebral Blood Flow Studies
CBF imaging techniques for moyamoya patients include PET,98 xenon CT,99,100 Xe,101–103 and SPECT104,105 Regional CBF in patients with moyamoya is characteristically diminished in the frontal and temporal lobes, and elevated in the posterior circulation territory (cerebellum and occipital lobes), and in central brain structures that are involved with basal moyamoya vessels. The degree of haemodynamic stress in patients with moyamoya disease varies greatly between patients.106 CBF studies can help predict the risk of stroke99 and the success of revascularization surgery.107
19.2.4.5 EEG
EEG findings are nonspecific in adults with moyamoya. Although EEG used to be a screening tool for children suspected of having moyamoya disease, it is currently considered to be somewhat obsolete for this purpose.90 Characteristic EEF findings may be observed in children with moyamoya disease. These include the following:108
1)
Low-amplitude slow waves, aka hemispheric posterior slowing or centrotemporal slowing.
2)
Sleep spindle depression.
3)
Rebuildup phenomenon: Hyperventilation characteristically causes a buildup of slow waves that resolves within 20–60 s after hyperventilation. In 70% of cases, buildup of slow waves appears after the original buildup had returned to baseline. Rebuildup of slow waves has been localized to the deep cortical sulci in regions of haemodynamic failure.109 This phenomenon disappears after surgery for moyamoya,110 and is not observed in adults with moyamoya.108
19.2.5 Clinical Features of Moyamoya Disease
The classic description of moyamoya disease separates the juvenile form from the adult form. This corresponds to the bimodal age distribution – with a higher peak around 5 years of age and a lower peak during the fourth and fifth decades – and the observation that younger patients tend to present with ischaemic symptoms and adult patients often present with haemorrhage. This distinction is appropriate for patients with moyamoya disease. Growing evidence suggests that the clinical expression of moyamoya syndrome, particularly in North American and European patients, is fundamentally different, in that ischaemia seems to be the most common presenting symptom, regardless of age.88–90 The clinical features of moyamoya syndrome are discussed separately below.
1)
Juvenile form:
a)
Initial signs and symptoms on admission:111
i)
Motor deficit: 81.5%
ii)
Headache: 27.2%
iii)
Mental retardation: 19.8%
iv)
Speech disturbance: 17.3%
v)
Sensory disturbance: 16.0%
vi)
Seizure: 6.2%
vii)
Involuntary movement: 6.2%
b)
Neurological symptoms are often precipitated by hyperventilating during activities such as crying or blowing.44
d)
Mental retardation has been described in >50% of cases, and the onset of ischaemic symptoms in patients <5-years old is associated with progressive mental retardation.112
e)
Symptoms in children often stabilize over time, as collateral circulation develops and an age-dependent decrease in CBF demand by the brain occurs.44
2)
Adult form:
a)
Intracranial haemorrhage is the presenting event in >60% of cases.88
i)
Bleeding may arise from the following:113
(1)
Abnormal vascular networks
(2)
Intracranial aneurysms
ii)
About 1/3 of patients presenting with haemorrhage will have another haemorrhage within days to years of the initial event.88
iii)
Intraventricular haemorrhage is the most common form of haemorrhage, present in 69% of patients presenting with haemorrhage.114
v)
Risk of rehaemorrhage: In a series of 42 patients with haemorrhagic moyamoya disease followed for a mean of 80.6 months, the average annual rebleeding rate was 7.09% per person.114 After rebleeding, the rate of good recovery fell from 45.5% to 21.4% and the mortality rate rose from 6.8% to 28.6%.
b)
Long-term clinical outcome:
i)
Overall, some 75% of the patients in Japan with moyamoya disease have normal activities of daily life or working ability, even prior to treatment.46
3)
Unilateral disease (aka probable moyamoya disease): There is some evidence that unilateral moyamoya disease is distinct from typical bilateral moyamoya disease.
a)
Get Clinical Tree app for offline access

Progression to bilateral disease:
i)




In an analysis of 180 cases of unilateral disease in Japan, only 7% developed into the definite type in an average follow-up period of 6.6 years.49
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree