CHAPTER 27 Intracranial Cysts and Cyst-Like Lesions
Non-neoplastic, noninfectious intracranial cysts are uncommon benign lesions of variable etiology and pathogenesis. The nomenclature for these lesions can be confusing. Single entities often have multiple names. Lesions with similar embryologic origins and histologic findings may be designated differently in different locations.
NEURENTERIC CYST
A neurenteric cyst is a benign malformative endodermal cyst of the central nervous system (CNS). Alternate names include enterogenous cyst, enteric cyst, endodermal cyst, gastroenterogenous cyst, gastrocytoma, intestinoma, and archenteric cyst.1
Epidemiology
Neurenteric cysts exhibit no age or gender predominance. They are rarely found intracranially, with fewer than 60 reported to date.2 Neurenteric cysts occur approximately three times more commonly in the spine than in the intracranial space.
Clinical Presentation
Intracranial neurenteric cysts most often present as headache (47.1%), then cranial nerve deficits (23.5%), new-onset seizure (17.6%), and recurrent meningitis (11.8%).3
Pathophysiology
A neurenteric cyst is thought to result from fusion of the ectoderm and endoderm before notochord formation, persistence of endodermal cells attached to the notochord during excalation, or partial regression of a duplicated neurenteric canal. These displaced nests of alimentary tissue ultimately form the neurenteric cyst.4
Neurenteric cysts may develop anywhere along the neural axis. Midline supratentorial neurenteric cysts may originate from Seesel’s pouch, an endodermal diverticulum that appears just posterior to the oropharyngeal membrane and anterior to the rostral end of the notochord.2 However, such an embryogenesis could not explain the neurenteric cysts that are found along the convexity supratentorially.
Pathology
Histologically, neurenteric cysts at all sites are subdivided into three groups by the structure of their wall.5 Simple type 1 cysts have a thin wall formed by a layer of stratified or pseudostratified cuboidal or columnar epithelium resting on a basement membrane. These are the most common. More complex type 2 and 3 cysts have additional mesodermal elements such as smooth muscle and fat (type 2) and ependymal or glial tissues (type 3).
Intracranial neurenteric cysts exhibit two major histologic patterns3:
1. The cyst is composed of pseudostratified, highly ciliated columnar epithelium, with areas of cuboidal epithelium. The cyst wall is notably poor in mucin-producing cells.
2. The cyst is composed of simple columnar epithelium with nonciliated cells, and the cyst wall is rich in mucin-producing cells.
Imaging
Intracranial neurenteric cysts are typically sharply demarcated, round to oblong lesions with smooth or lobulated margins. Size varies from 1 to 9 cm. Supratentorial cysts are generally larger than their posterior fossa counterparts.3
Most intracranial neurenteric cysts (72%) are extra-axial masses of the low posterior fossa, especially the cerebellopontine angle and the midline pontomedullary junction. About 28% of intracranial neurenteric cysts are supratentorial. These are commonly related to the frontal lobes and most frequently lie away from the midline.3 Neurenteric cysts have also been found in the septum pellucidum, third ventricle, parasellar region, orbital apex, superior orbital fissure, optic nerves, and oculomotor nerves. Intraventricular neurenteric cysts are very rare.3 Unlike spinal neurenteric cysts, intracranial neurenteric cysts are rarely associated with bony anomalies.
MRI
On MRI the cysts show equally variable signal intensity. Most neurenteric cysts are hyperintense on fluid-attenuated inversion recovery (FLAIR) sequences. Highly proteinaceous cysts are typically isointense to slightly hyperintense to CSF on T1W images and typically hyperintense on T2-weighted (T2W) images. Cysts with marked squamous metaplasia of the epithelium and voluminous keratinous material within the lumen are often hypointense on T2W images.3 Neurenteric cysts usually do not enhance. However, minimal rim enhancement may result when a cyst wall adheres to brain parenchyma.3 DWI and apparent diffusion coefficient (ADC) maps of neurenteric cysts show absent or slight restriction of diffusion in most cases, but there is substantial variability among cases.3
KEY POINTS: DIFFERENTIAL DIAGNOSIS OF NEURENTERIC CYST
 Epidermoids have incomplete signal suppression on FLAIR images and hyperintensity on diffusion-weighted trace images.
Epidermoids have incomplete signal suppression on FLAIR images and hyperintensity on diffusion-weighted trace images.
 Dermoid cysts have high signal intensity similar to that of fat that helps to differentiate them from neurenteric cysts.
Dermoid cysts have high signal intensity similar to that of fat that helps to differentiate them from neurenteric cysts.
 The presence of an enhancing mural nodule that represents a scolex and an enhancing rim are helpful in the diagnosis of neurocysticercosis.
The presence of an enhancing mural nodule that represents a scolex and an enhancing rim are helpful in the diagnosis of neurocysticercosis.
 In cystic tumors, such as a schwannoma or meningioma, clear peripheral or intratumoral enhancement is present.
In cystic tumors, such as a schwannoma or meningioma, clear peripheral or intratumoral enhancement is present.
 Differentiation of arachnoid cyst from a neurenteric cyst is difficult when the neurenteric cyst has the same signal intensity as CSF.
Differentiation of arachnoid cyst from a neurenteric cyst is difficult when the neurenteric cyst has the same signal intensity as CSF.
RATHKE’S CLEFT CYST
Rathke’s cleft cyst is a non-neoplastic cyst arising from remnants of the embryonic Rathke’s cleft.
Epidemiology
Rathke’s cleft cysts are found in 2% to 26% of pituitary glands studied at autopsy. Incidental cysts were found in the pituitary gland in 113 (11.3%) of 1000 nonselected autopsy specimens.6
Clinical Presentation
Most Rathke’s cleft cysts are asymptomatic. A few cause symptoms by compression of intrasellar or suprasellar structures, leading to hypopituitarism, amenorrhea, galactorrhea, headache, and visual field defects. Rarely, symptoms result from hemorrhage into or around the cyst.7
In 53 symptomatic patients with Rathke’s cleft cysts, headache was the most common presenting symptom (81%) and was often accompanied by other symptoms.8 Other findings were visual impairment (47%); hormonal abnormalities such as hyperprolactinemia, hypocortisolism, hypothyroidism, hypogonadism, or panhypopituitarism (38%); and other endocrine disturbances such as diabetes insipidus, galactorrhea, amenorrhea, or oligomenorrhea (30%).8 Pituitary apoplexy was present in 4 patients (7.5%).
Pathophysiology
Rathke’s cleft cysts are thought to result from failure to obliterate the lumen of Rathke’s pouch. Rathke’s pouch normally develops as a rostral diverticulum of the primitive oral cavity during the third or fourth weeks of gestation. It has an anterior wall, a posterior wall, and a central embryonic cleft. The anterior wall of the pouch proliferates to form the anterior lobe of the pituitary gland and the pars tuberalis. The posterior wall becomes the pars intermedia. The residual lumen of the pouch is reduced to a narrow Rathke’s cleft, which generally regresses. Persistence and enlargement of this cleft is thought to cause the cyst.9 The epithelium of the cyst is a vestige of Rathke’s pouch.
Pathology
Rathke’s cleft cysts are round to ovoid and vary from 2 to 40 mm. The capsule is frequently thin and transparent but may be gray, blue, or white. Cyst contents vary from clear material similar to CSF to purulent or pigmented fluid and have a variable watery, caseous, or inspissated consistency.8 Pigmented cyst contents are common and together with the white and purulent ones are present in 78% of patients.
Rathke’s cleft cysts have a smooth inner wall, unless inflammation or hemorrhage causes irregularity and opacity. There is no intrinsic epithelial soft tissue mass within the cyst. However, solid polysaccharide material may form intracystic nodules that mimic a soft tissue within the cyst.10
The wall of the Rathke cleft cyst is a single layer of cells resting on a basement membrane. The epithelium is most commonly pseudostratified columnar epithelium. It is often ciliated and may contain goblet cells. Squamous epithelium, including focal squamous metaplasia, is noted in 23% of cases. Hemosiderin pigmentation, other blood products, and cholesterol crystals are common.8 Signs of chronic inflammation are present in 32% of cysts, but calcifications are unusual.
Imaging
Rathke’s cleft cysts typically appear as well-demarcated rounded or ovoid intrasellar-suprasellar lesions located exactly in the midline between the anterior and posterior pituitary lobes (Fig. 27-1). They usually exhibit a smooth contour, homogeneous density/signal intensity within the lesion, and no calcification. Blood products and debris may, however, cause inhomogeneity of cyst texture and signal. Contrast enhancement within the surrounding pituitary gland often delimits the cyst as a filling defect within the gland, but the cyst contents show no enhancement.
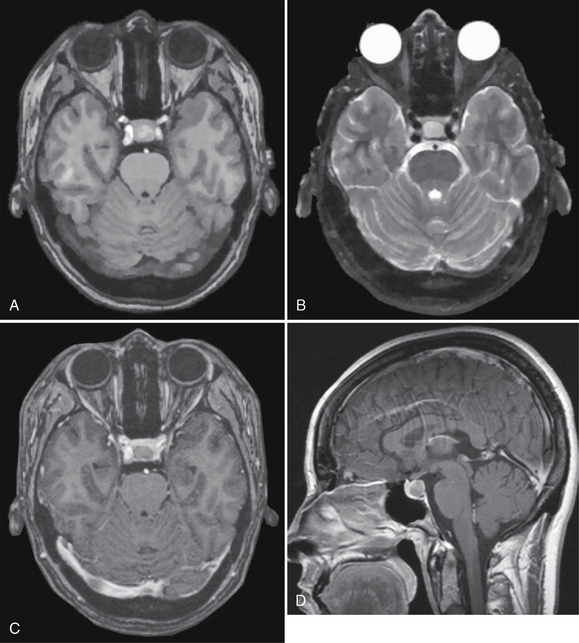
FIGURE 27-1 Rathke’s cleft cyst. Axial T1W (A) and T2W (B) MR images and postcontrast axial (C) and sagittal T1W (D) MR images show a well-demarcated intrasellar ovoid lesion located at the midline between the anterior and posterior pituitary lobes. The cyst is slightly hyperintense on the T1W image (A) and does not enhance.
Rathke’s cleft cysts range in size from 5 to 40 mm (mean, 17 mm). Only 40% are completely intrasellar; 60% to 70% involve both intrasellar and suprasellar regions simultaneously (Fig. 27-2). Atypical locations include purely suprasellar, intrasphenoid, and nasopharyngeal sites (Fig. 27-3).
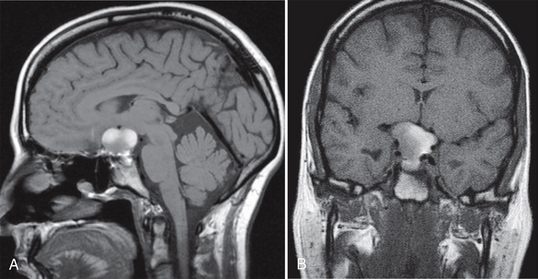
FIGURE 27-2 Rathke’s cleft cyst with sellar and suprasellar component. Sagittal (A) and coronal (B) noncontrast T1W MR images show a predominantly hyperintense sellar mass with suprasellar extension.
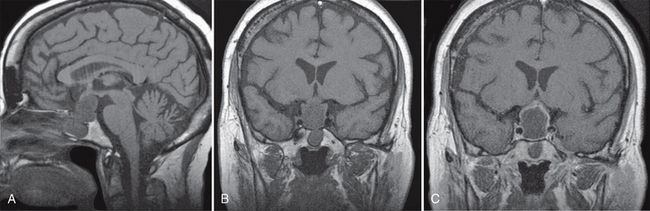
FIGURE 27-3 Intrasphenoid, sellar, and suprasellar Rathke’s cleft cyst. Sagittal (A) and coronal (B) noncontrast T1W MR images demonstrate a hypointense mass involving the sphenoidal sinus, pituitary fossa, and suprasellar regions. Postcontrast coronal T1W MR image (C) shows minimal wall enhancement.
CT
On CT these cysts show highly variable density. A retrospective study of 23 patients with symptomatic Rathke’s cleft cysts, showed the cyst was isodense with respect to gray matter in 30%, was hypodense in 48%, and had high density in 22%.8 Cyst wall calcification is unusual. Nodular or rim enhancement is uncommon.
MRI
As on CT, Rathke’s cleft cysts are similarly variable on MRI, because the MR signal depends on the presence and concentration of cyst protein, mucopolysaccharides, chronic hemorrhage, cholesterol, and cellular debris from the cyst wall. High concentrations of these materials shorten the T1 and T2 relaxation times, leading to high signal intensity on T1W and low signal intensity on T2W imaging.
Rathke’s cleft cysts can be classified into three types on the basis of their signal intensity. Type 1 cysts show low signal intensity on T1W images and high signal intensity on T2W images.11 This is the most common group. Type 2 cysts show high signal intensity on both T1W and T2W images. The amount of mucopolysaccharide in the mucoid material influences the signal intensity in type 2 cysts. This is the second most common group. Type 3 cysts show high signal intensity on T1W images and low signal intensity on T2W images. This pattern most likely results from prior hemorrhage.12 Small cysts are usually homogeneous in signal intensity, whereas other lesions such as cystic craniopharyngiomas and hemorrhagic adenomas more frequently have heterogeneous signal intensity.
The wall of the cyst shows thin rim enhancement in 28% of lesions studied by MRI but only 9% studied by CT.8 This rim has been ascribed, variably, to compressed, peripherally displaced pituitary tissue or to changes due to squamous metaplasia, inflammation, or deposition of hemosiderin or cholesterol crystals.
Like CSF, Rathke’s cleft cysts appear hypointense on echoplanar DWI.13 ADC maps show markedly increased ADC in all cases. In one report, the average ADC value of Rathke’s cleft cysts was 2.12 ± 0.29 (range, 1.60-2.48) × 10−3 mm2/s and the average relative ADC of Rathke’s cleft cysts was 2.61 ± 0.37 (range, 1.95-3.18), a value similar to that of the CSF.14
KEY POINTS: DIFFERENTIAL DIAGNOSIS OF RATHKE’S CLEFT CYST
 Craniopharyngiomas are mostly suprasellar. Rathke’s cleft cysts are typically intrasellar.
Craniopharyngiomas are mostly suprasellar. Rathke’s cleft cysts are typically intrasellar.
 Rathke’s cleft cysts do not typically enhance. Enhancing solid components are present in craniopharyngiomas.
Rathke’s cleft cysts do not typically enhance. Enhancing solid components are present in craniopharyngiomas.
 Calcification is found frequently in craniopharyngiomas but infrequently in Rathke’s cleft cysts.
Calcification is found frequently in craniopharyngiomas but infrequently in Rathke’s cleft cysts.
 As compared with pituitary adenoma, Rathke’s cleft cysts seldom extend beyond the lateral wall of the internal carotid artery.
As compared with pituitary adenoma, Rathke’s cleft cysts seldom extend beyond the lateral wall of the internal carotid artery.
COLLOID CYST
Colloid cysts are congenital benign epithelium-lined cysts that nearly always arise in the anterior third ventricle. The name derives from the Greek word kollodes, resembling glue, in reference to the cyst’s contents. It is also called a paraphyseal cyst.
Epidemiology
Colloid cysts are found in approximately three individuals per million per year and account for approximately 1% of all intracranial masses.15 They are the most common intracranial neuroepithelial cysts and most common third ventricular tumor. Most colloid cysts present in the third to fifth decades of life. Fewer than 40 cases have been described in children.16 No gender predilection is known. Rare cases of familial colloid cysts are reported.17
Clinical Presentation
Most colloid cysts are asymptomatic and found incidentally. In symptomatic patients, the most common complaint (68%-100%) is headache that is initiated, exacerbated, or relieved by a change in position and lasts from seconds to minutes.18 Other symptoms include progressive dementia, cognitive impairment, and spells of transient loss of consciousness, related to hydrocephalus. In children, the most common symptoms are headache, nausea, vomiting, papilledema, and diplopia.19
Colloid cysts may cause acute hydrocephalus and sudden death.20 Such acute deterioration may result from downward movement of the cyst after lumbar puncture.21 In rare cases, hemorrhage into the cyst may cause acute enlargement of the cyst and obstruct CSF flow.22 Cyst size is not a reliable predictor of outcome, because even small cysts may cause sudden death.23
Pathophysiology
Colloid cysts originate when ectopic endodermal elements migrate into the velum interpositum during development of the CNS.24 Immunohistochemically, colloid cysts are similar to the respiratory mucosa of the trachea and sphenoidal sinus, suggesting an endodermal origin.24 Ultrastructural analysis reveals ciliated cells, nonciliated cells with microvilli, goblet cells with secretory granules, basal cells, and undifferentiated cells with scanty organelles. The type and topographic arrangement of the cells again resemble respiratory epithelium and suggest that colloid cysts have an endodermal lineage.25 For these reasons, some consider colloid cysts, neurenteric cysts, and Rathke’s cleft cysts to represent the same lesion at different locations.26
Pathology
Histologically, colloid cysts show an inner lining of low cuboidal-pseudostratified columnar epithelial cells with occasional ciliated and goblet cells resting on a thin capsule of fibrous connective tissue. The cyst contains amorphous, faintly eosinophilic material that is strongly periodic acid–Schiff (PAS) positive owing to the presence of glycogen and mucosubstances. There may be recent or remote hemorrhage and cholesterol crystals. Chemical irritation from hemorrhage, cholesterol, or the colloid material itself may give rise to a secondary xanthogranulomatous reaction within the cyst wall.27
Imaging
The most nearly characteristic feature of colloid cysts is their location in the anterior third ventricle. Rarely, however, true colloid cysts are found within the lateral ventricles, fourth ventricle, brain stem, prepontine cistern, suprasellar region, and cerebral convexity. The cysts are typically smooth-walled, rounded masses measuring 3 to 40 mm in diameter (occasionally larger) (Figs. 27-4 and 27-5).
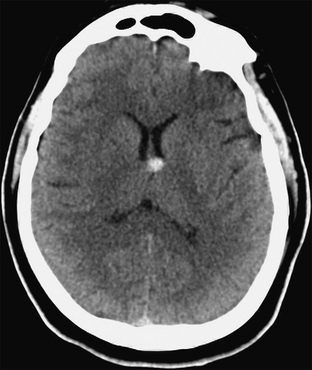
FIGURE 27-4 Colloid cyst. Axial CT without contrast enhancement shows a hyperdense rounded nodule in the anterior aspect of the third ventricle. No hydrocephalus is present.
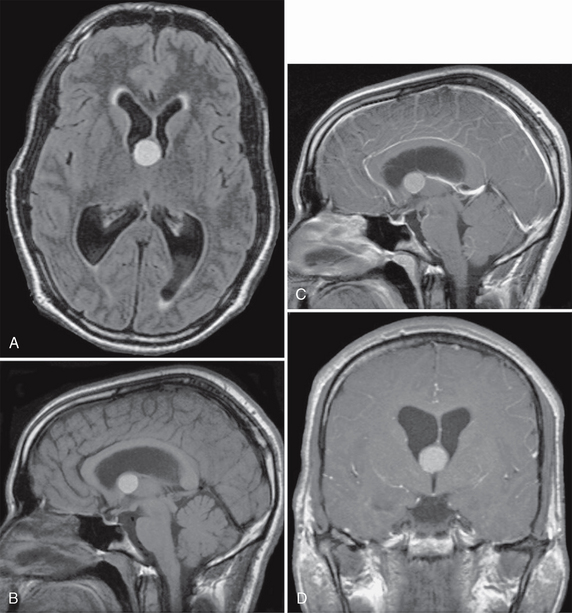
FIGURE 27-5 Colloid cyst with obstructive hydrocephalus. Axial FLAIR (A) and sagittal (B) T1W MR images show a hyperintense nodular mass at the foramen of Monro. Postcontrast sagittal (C) and coronal (D) T1W MR images demonstrate no obvious enhancement of the lesion. Dilatation of both lateral ventricles is due to obstruction of CSF flow at the foramen of Monro.
CT
Most colloid cysts are slightly hyperdense with respect to the brain, but they may appear hypodense or isodense (see Fig. 27-4).28 Contrast enhancement may show a thin enhancing rim that is thought to represent the cyst capsule. Calcifications are rare. Because CT density reflects the hydration state of the cysts, colloid cysts that are hypodense on CT scans (watery) may be aspirated successfully29 whereas cysts that are hyperdense on CT (and therefore more viscid) are likely to fail stereotactic aspiration.
MRI
About 50% of colloid cysts are hyperintense on T1W images. The rest are either isointense or hypointense with respect to brain. On T2W imaging, most colloid cysts are isointense to hypointense with respect to the brain. Most commonly, colloid cysts appear hyperintense on T1W images and isointense to hypointense on T2W images. Although the central T2 hypointensity was believed to result from hemorrhage and the paramagnetic effects of metal ions within the fluid, more recent studies suggest that cholesterol contents and paramagnetic substances are responsible for the MRI appearance.19 Isointense cysts may be more difficult to detect with MR images as compared with CT scans.22 Some cysts nearly indetectable on T1W and T2W images are readily displayed on proton density–weighted images. Occasionally intracystic fluid levels are present. Vascularity in the outer cyst wall may appear as a thin rim of peripheral enhancement, However, central enhancement suggests an alternate diagnosis of solid tumor, because colloid cysts do not contain intrinsic central soft tissue. On DWI, colloid cysts may show elevated water diffusion.30 It is expected that DWI and ADC maps will ultimately show the same variations in cyst hydration already known from pathology, CT, and MRI.
KEY POINTS: DIFFERENTIAL DIAGNOSIS OF COLLOID CYST
 A hyperdense nodular mass at the foramen of Monro suggests a diagnosis of colloid cyst. However, craniopharyngiomas of the middle third of the ventricle and aneurysms of the basilar tip must be excluded.
A hyperdense nodular mass at the foramen of Monro suggests a diagnosis of colloid cyst. However, craniopharyngiomas of the middle third of the ventricle and aneurysms of the basilar tip must be excluded.
 Neoplasms of the anterior third of the ventricle such as choroid plexus tumors, meningiomas, and gliomas typically show enhancement.
Neoplasms of the anterior third of the ventricle such as choroid plexus tumors, meningiomas, and gliomas typically show enhancement.
GLIOEPENDYMAL CYST
This is a benign epithelial-lined CNS cyst that is also referred to as a neuroglial cyst.
Pathophysiology
The origin of glioependymal cysts is uncertain. Supratentorial intraparenchymal glioependymal cysts may arise from the lining of the embryonic neural tube, sequestered in some way within the developing white matter. Subarachnoid glioependymal cysts may derive from subarachnoid neuroglial heterotopias.31 Posterior fossa glioependymal cysts are thought to arise from remnants of Blake’s pouch32 (a diverticulum of the roof of the fourth ventricle)33 or from the tela choroidea.34 Glioependymal cysts may be associated with agenesis of the corpus callosum, gray matter heterotopias, and other cortical dysplasias.
Pathology
Grossly, these cysts are smooth, round, unilocular cysts filled with clear fluid resembling CSF.
Imaging
Glioependymal cysts appear anywhere along the neural axis but are more common in the frontal lobes. They are rounded, smooth, unilocular cysts ranging in size from a few millimeters to several centimeters. They contain clear fluid that resembles CSF (Figs. 27-6 and 27-7).
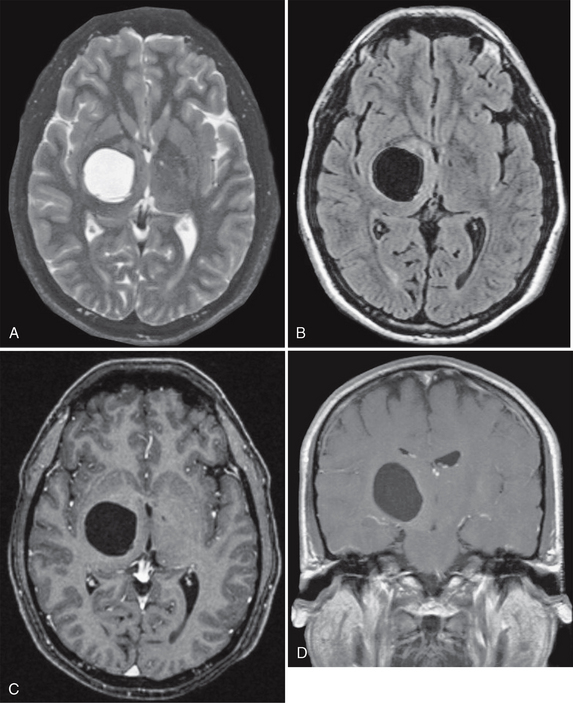
FIGURE 27-6 Presumed glioependymal cyst. A cystic mass that is hyperintense on axial T2W MR image (A) and hypointense on FLAIR (B) imaging is found in the right thalamic region. Postcontrast axial (C) and coronal (D) T1W MR images demonstrate no enhancement of the lesion.
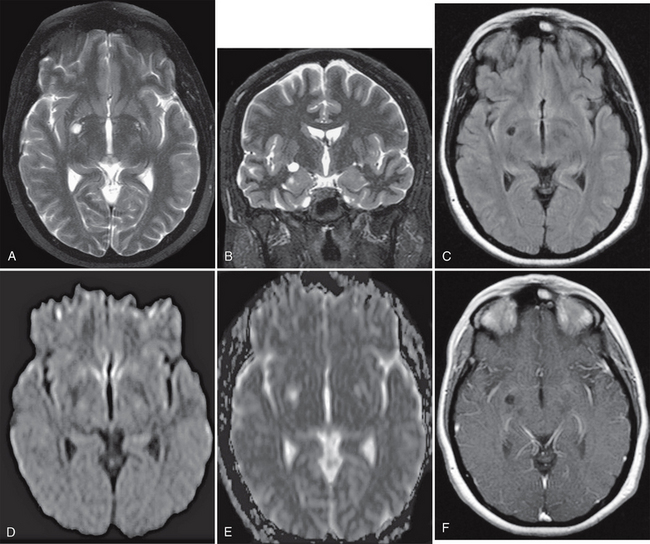
FIGURE 27-7 Presumed glioependymal cyst. A small, right basal ganglia cystic lesion that is hyperintense on axial (A) and coronal (B) T2W MR images and hypointense on axial FLAIR image (C) is seen. On DWI, the cyst is hypointense on the DW trace image (D) and hyperintense on the ADC map (E), indicating elevated water diffusivity. Postcontrast axial T1W MR image (F) demonstrates no enhancement of the lesion.
MRI
KEY POINTS: DIFFERENTIAL DIAGNOSIS OF GLIOEPENDYMAL CYST
 Enlarged perivascular spaces are typically multiple and clustered around the anterior commissure in the lateral basal ganglia. The frontal lobes are the most typical location for glioependymal cysts.
Enlarged perivascular spaces are typically multiple and clustered around the anterior commissure in the lateral basal ganglia. The frontal lobes are the most typical location for glioependymal cysts.
 Neurocysticercosis can partially enhance and has a discrete eccentric scolex. Glioependymal cysts do not enhance.
Neurocysticercosis can partially enhance and has a discrete eccentric scolex. Glioependymal cysts do not enhance.
 Cystic periventricular leukomalacia is characterized by marked loss of periventricular white matter, predominantly in the periatrial regions, and shows atrophic ventricular enlargement adjacent to regions of abnormal white matter.
Cystic periventricular leukomalacia is characterized by marked loss of periventricular white matter, predominantly in the periatrial regions, and shows atrophic ventricular enlargement adjacent to regions of abnormal white matter.



