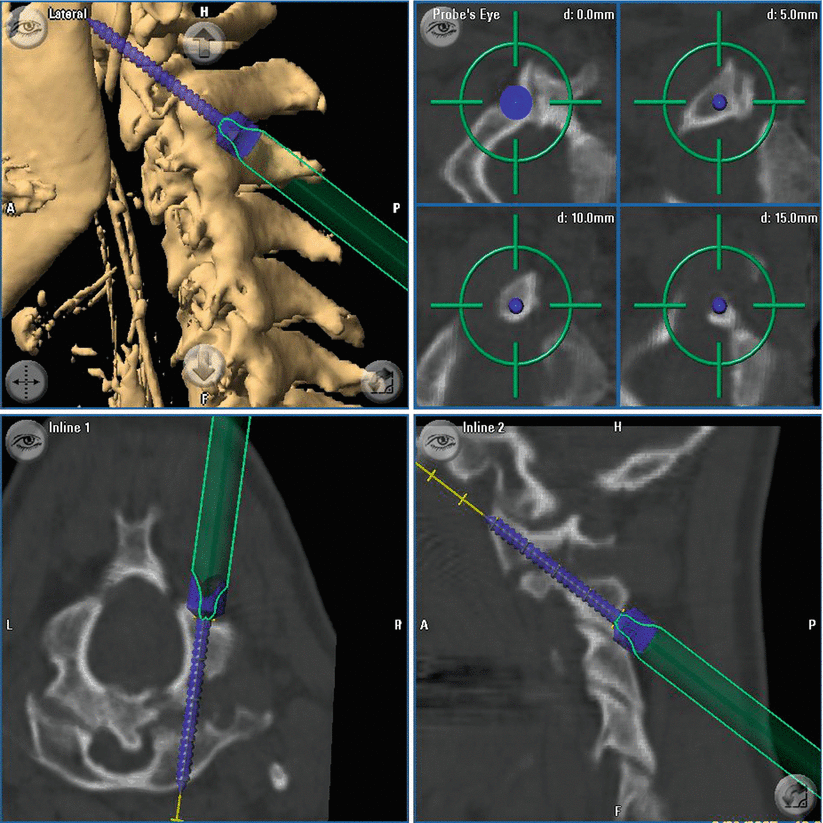
Fig. 39.1
Set-up of ceiling-mounted navigation system, radiolucent OR table and multislice CT scanner with a sliding gantry
Cranial CT and CTA/CT Perfusion
Pre- and intraoperative imaging in cranial surgery was performed using a collimation of 24 × 1.2 mm, in pituitary surgery of 40 × 0.6 mm at 120 kV, 320 mAs, pitch 0.9 and a rotation time of 1 s. Reconstructions were performed with a slice thickness of 0.6, 1.0, 1.5, 2 and 5 mm. If necessary contrast media were given via an automated infusion pump (Stellant MEDRAD Inc., Indianola, Pennsylvania, USA) with a weight-adapted contrast agent injection protocol followed by a saline chaser (1.4 ml contrast agent per 1 kg bodyweight Imeron 300 (Bracco-Altana Pharma, Konstanz, Germany) and 0.42 g iodine per 1 kg bodyweight at an injection rate of 0.035 × bodyweight ml/s followed by 100 ml saline at 0.035 × bodyweight ml/s, respectively). For CT angiography a collimation of 40 × 0.6 mm was used at 120 kV, 120 mAs, pitch 1.1 and rotation time of 1 s. Contrast agent injection was done by using an automated infusion pump with a dedicated protocol (1.35 ml contrast agent per 1 kg bodyweight Imeron 300 (Bracco-Altana Pharma, Konstanz, Germany) and 0.4 g iodine per 1 kg bodyweight at an injection rate of 6.0 ml/s followed by 100 ml saline at 6.0 ml/s, respectively). For image reconstructions a slice thickness of 1.0 mm and an increment of 0.75 mm were used. Multiplanar reconstructions; maximum intensity projections in axial, coronal and sagittal orientations; and reconstructions using volume-rendering technique were used. For cerebral perfusion measurements a standard contrast agent injection protocol was used, and one scan was performed every second over a time period of 40s using 80 kV and 200 mAs with a collimation of 24 × 1.2 mm. Perfusion scanning started 5 s after injection of 50 ml contrast agent (Imeron 300) at 7 ml/s and a saline flush of 50 ml at 7 ml/s. After acquisition standard perfusion analysis was performed using a standard workstation (Syngo MMWP, Siemens Medical Solutions, Erlangen, Germany) and colour-coded parameter maps of cerebral blood flow, cerebral blood volume and time to peak were calculated Fig. 39.3.
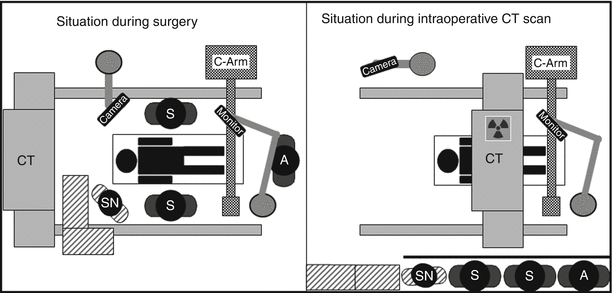

Fig. 39.2
Schematic drawing of intraoperative set-up during surgery. A anaesthesia, SN scrub nurse, S surgeon, A assistant
Spinal Imaging
In spinal surgery a non-enhanced protocol was used for spondylodesis and a contrast-enhanced protocol for tumour surgery. Scans were performed using a collimation of 24 × 1.2 mm at 120 kV, 240 mAs, pitch 0.9 and a rotation time of 1 s, using automated dose-modulation software. MPR reconstructions with a slice thickness of 3 mm were calculated in axial and sagittal orientations.
Study Population
Beginning January 2006 until March 2008, 240 neurosurgical patients with a mean age of 55 ± 17 years were operated with the help of iCT. There were 146 cranial (105 tumours, 10 patients with unruptured aneurysms) and 94 navigated spinal stabilisation operations. As the system was used in a multidisciplinary OR, trauma surgery was also performed but is not reported here. In cranial surgery, iCT was used for automated navigation, intraoperative control of tumour resection, intraoperative CT angiography and measurement of cerebral blood flow in aneurysm surgery. In spinal surgery, the system was predominantly used for navigated instrumentation and intraoperative control of correct placement of screws and other implants.
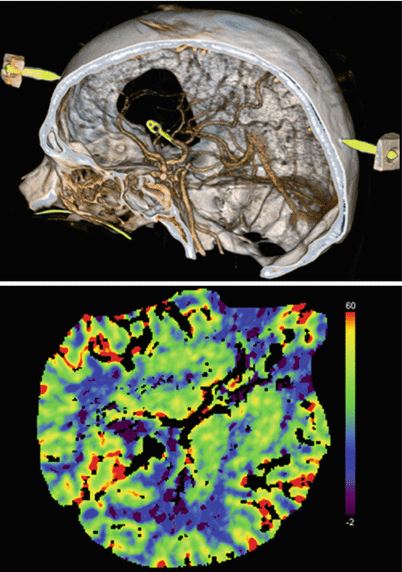

Fig. 39.3
Intraoperative CT angiography and normal perfusion CT after clipping of a right-sided MCA aneurysm. There are no artefacts caused by trepanation borders
Analysis
Surgical and anaesthesiological set-up, intraoperative workflow, technical problems of iCT and integration of the navigation system were recorded. Times required for preoperative scanning (preoperative CT, safety checkup, data transfer, registration of navigation system) and intraoperative scanning (sterile draping, scan, resumption of surgery) for each procedure were assessed by a person not directly involved in the surgical procedure. Quality of intraoperative imaging with special regard to artefacts was assessed. Change of surgery based on intraoperative imaging was documented for each procedure. An intraoperative CT was obtained to assess the extent of decompression and the accuracy of instrumentation. All screws were evaluated on multiplanar reconstructions and rated on the basis of a 2-mm increment classification suggested by Gertzbein and Robbins [5].
Results
Workflow
Patients are positioned on the operating table in the position desired by the surgeon with no restrictions (supine, lateral, prone position) except the sitting position which can be used with the table, but scanning of a sitting patient cannot be performed. After final positioning of the patient according to the envisioned procedure, a “safety check” was performed, and the optimal scanning position of the operating table is saved in a computer panel at the wall allowing exact automatic repositioning for intraoperative imaging during surgery. Hereby the gantry is moved over the patient in order to detect any possible collision during the scanning procedure. In order to avoid artefacts in cranial surgery, optimal fixation of the pins of the radiolucent head clamp is of major importance and can require considerable time in some cases. After mounting of the reference clamp, automated registration of the navigation system is performed, and the CT data can optionally be matched with pre-existing MRI data. Both data sets can then be used for navigation. For an intraoperative update of imaging and neuronavigation, the surgical procedure is stopped, and all metal material is removed from the surgical site. The instrument tables and the microscope are moved outside the sliding area of the CT scanner, and the patient is covered with a sterile drape. The OR table is moved automatically to the initially saved position using the remote control. Contrast media may be given either manually as bolus, by infusion or using an automated infusion pump. In all study subjects we did not find any contrast agent injection-related minor or major adverse event, like extravasation, allergic or anaphylactic reactions or impairment of renal function (serum creatinine test at postinterventional, routine blood samples 2–7 days after surgery). There was no collision of the patient and the scanner or any new neurological deficit or infection related to the intraoperative scanning.
In case of spinal instrumentation, preoperative imaging was performed at that time with the data fed into the navigation. Hereby, the real position of the spine during surgery is the basis for navigation. This is especially valuable if luxation of the spine has to be reduced in the OR (e.g. in case of cervical instability) since any navigation on the basis of image sets obtained prior to the positioning of the patient on the OR table may be misleading. For spinal navigation the reference star is attached to the spinous process of the vertebra intended to be navigated. Then surface or region-based registration is accomplished – the most recent software release allows automated registration without manual scanning of the surface. In this case generation of images for navigation and registration is accomplished with one intraoperative scanning procedure. After placement of the screws, an intraoperative control-CT scan is performed. For intraoperative imaging during surgery, the patient is covered with a sterile drape, and the scan is performed as described above. Misplaced screws are corrected immediately followed by another intraoperative control scan. As this is a high-resolution CT, there is no need to perform a postoperative CT as image quality would not be better.
Time Requirements
Compared to similar cranial surgical interventions without iCT, additional time needed for preoperative positioning, safety check, preoperative iCT, data transfer to the navigation system and referencing is 22 ± 5 min. Time for intraoperative scans in cranial surgery depends mainly on whether application of contrast media is needed which requires additional time. In general, an intraoperative cranial CT scan takes approximately 10–15 min (sterile draping, scan, resumption of surgery) from the point of time when surgery is stopped until surgery is resumed. In spinal surgery, time needed for preoperative image acquisition including safety checkup and data transfer was 14 ± 5 min. The time-out for intraoperative scanning until continuation of surgery was 9 ± 2.5 min ranging from 4 to 15 min.
Evaluation of Cranial Imaging
As expected, iCT for control of tumour resection is especially useful in osseous tumours and contrast-enhancing tumours such as meningiomas (especially sphenoid wing meningiomas), metastases or high-grade gliomas. In low-grade gliomas iCT has to be refined further due to the low soft-tissue contrast. In pituitary surgery iCT is sufficient to detect tumour remnants in macroadenomas but is not found useful for imaging of microadenomas. Furthermore, iCT evolved to be very useful in cases of difficult placement of ventricular catheters (e.g. for shunt surgery), allowing navigated positioning and immediate control of position of the catheter and replacement, if necessary.
In 9 of 10 dynamic perfusion CT data sets, the quality was rated excellent or good. In the remaining case, the quality was rated insufficient for diagnostic evaluation due to major streak artefacts induced by the titanium pins of the head clamp. In this particular case, the quality of the related CT angiography was rated good and sufficient for intraoperative decision-making. The quality of all 12 CT angiography data sets was rated excellent or good. In one patient with an anterior communicating artery aneurysm, PCT scanning led to a repositioning of the clip because of an ischemic pattern of the perfusion parameter maps due to clip stenosis of an artery. The subsequent PCT scan obtained in this patient revealed an improved perfusion of the related vascular territory, and follow-up MR imaging showed only minor ischemia of the anterior cerebral artery territory.
Evaluation of Spinal Imaging
With CT-guided spinal navigation, a computed spatial accuracy of 0.8. ± 0.1 mm could be achieved. Control-iCT revealed incorrect screw position ≥2 mm with violation of the pedicle cortex in 20/414 screws (4.8 %). In 65/414 of the screws (15.7 %) we detected a minor pedicle perforation <2 mm. In 75 % of those screws the diameter of the perforation was equal or under the distance of the screw thread (≤1 mm). Due to the small pedicle configurations, the highest rate of cortical breaches was seen in the mid-thoracic region (T5–T8) with 27.5 % (14/51 screws). In four of those screws, the perforation was ≥2 mm. In the whole series there was no evidence for blowout fractures of the pedicle. Intraoperative control imaging changed the course of surgery in 8 cases (8.5 % of all patients) with immediate correction of 10 screws (2.4 %) without any persistent damage to nerves or vessels. The remaining 10 screws were classified sufficient regarding stability and position towards endangered anatomical structures. In addition, in case of patients with tumorous disease, the extent of tumour resection could be confirmed. There was no reoperation due to implant malposition. There was no increase of infection rate or other procedure-related morbidity compared to patients from the pre-iCT era. Time needed for the stabilisation procedure (surgical exposure, registration of the spine, screw placement and intraoperative control-iCT) was 83.1 ± 22.4 min (2 screws) for transarticular C 1/2, 77.2 ± 24.1 min for lumbar (4 screws) and 102.7 ± 25.0 min (8 screws) for thoracic transpedicular screw placement Fig. 39.4.