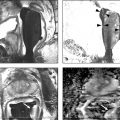
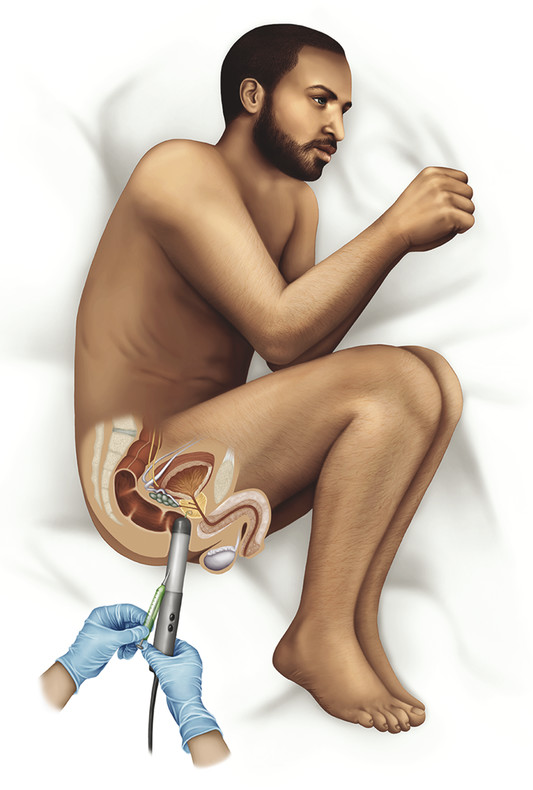
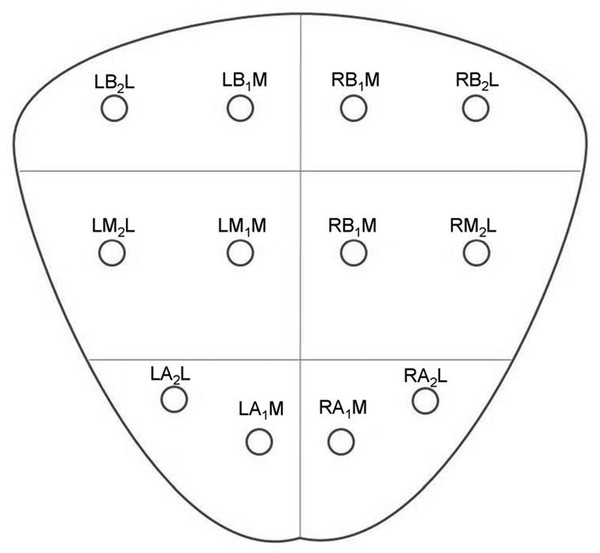
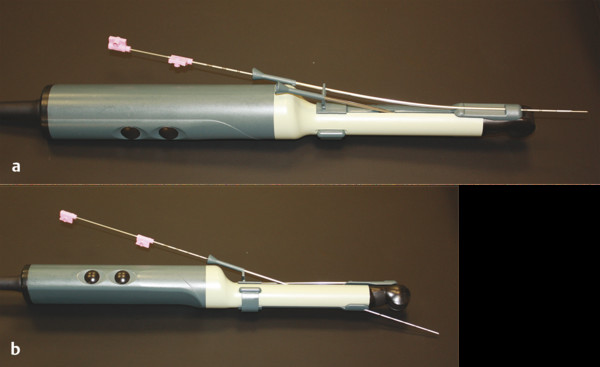
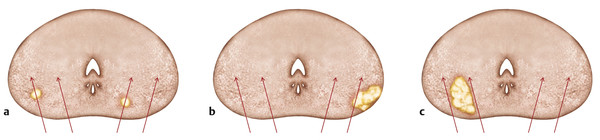
Incidence, Demographics, and Survival Prostate cancer is the second most common cancer in men in the United States, after only skin cancer. After lung cancer, prostate cancer is the second leading cause of cancer death in American men. It is estimated that there are nearly 3 million American men currently living with prostate cancer. 1 Prostate cancer alone accounted for about one-quarter of new cancer diagnoses in men, with 220,800 new diagnoses and 27,540 estimated deaths in 2015 alone . 2 Approximately 14% of men (1 in 7) will be diagnosed with prostate cancer at some point during their lifetime, with a median age at diagnosis of 66 years, and about 1 in 38 men will die of prostate cancer. 1 For men with prostate cancer, 80.4% are diagnosed at the local stage. 1 Although the 5-year relative survival rate for men diagnosed in the United States from 2001 to 2007 with local or regional disease was 100%, the rate for distant disease was 28%. 1 Prostate cancer is a major public health concern and is associated with significant health-care costs. With an estimated increase in the elderly population from 400 million individuals > 65 years in 2000 to approximately 1.5 billion by 2050 3 coupled with an increase in the 10-year relative survival rate of those diagnosed with prostate cancer, the economic burden of prostate cancer is predicted to increase markedly. 4 Earlier detection through prostate-specific–antigen (PSA) screening has been successful in identifying men who might benefit from treatment. As a result, many men are now diagnosed earlier and with lower-stage cancer than was previously the case effectively increasing the economic burden of this disease. 4, 5, 6 In the Unites States, the total estimated expenditure on prostate cancer was nearly $10 billion in 2006, approximately $12 billion in 2010, and projected to reach almost $20 billion by 2020. 7 The mean annual costs per patient were $10,612 in the initial phase after diagnosis, $2,134 for continuing care, and $33,691 in the last year of life. 8 Patterns of costs vary widely based on initial treatment. Watchful waiting is reported to have the lowest initial treatment cost ($4,270). In comparison, initial treatment costs are highest for combined hormonal and radiation therapy ($17,474), as well as for surgery ($15,197). Five-year total costs are highest for hormonal therapy alone ($26,896), followed by combined hormonal and radiation therapy ($25,097), and then by surgery ($19,214). 9 However, improved paradigms for the clinical management of prostate cancer could greatly decrease these costs, specifically for men with low-risk indolent disease whose life expectancy will be not be affected by prostate cancer. For instance, the cumulative annual cost attributable to the “overtreatment” of prostate cancer in the United States has reached nearly $60 billion. Furthermore, the ability to not treat the 80% of men with low-grade disease who will never die of prostate cancer is estimated would save $1.32 billion per year nationally. 10 Improved identification of risk factors could help guide risk-adapted screening and preventive interventions. Both modifiable lifestyle factors and preventive therapies exist that might reduce the risk of developing prostate cancer. Age is the most important nonmodifiable factor. In unscreened populations, prostate cancer has the steepest age–incidence curve of all cancers, showing a rapid increase in the seventh decade. Racial variation is also significant. In the United States, compared with white men of European ancestry, black men of African ancestry have 58% greater incidence of prostate cancer and 144% greater mortality, whereas Hispanic men have 14% lower incidence and 17% lower mortality. 11 The relative risk of developing prostate cancer is approximately 2.5 times greater in men who have a first-degree relative with prostate cancer. Family history is important, although only 35% of the familial risk is explained by known genes. 12 Exposure to a variety of external agents may also play a role in the development of prostate cancer. First, smoking is associated with a moderately increased risk for prostate cancer. 13 The association between prostate cancer and smoking is much stronger, especially for aggressive cancers, in heavy smokers. 14 In addition, obesity is associated with a significantly increased risk for both low- and high-risk prostate cancer in black men. 15 However, among white men, the link between obesity and prostate cancer is less clear. Although no links with specific dietary factors have been definitively established, red meat, dairy protein, dietary fat, and coffee have all been postulated to serve as risk factors. 16 Finally, although the role of inflammation in prostate cancer carcinogenesis remains controversial, the risk for prostate cancer may be increased in men with a history of urinary tract infections 17 as a result of chronic intraprostatic inflammation. 18 In most cases, prostate cancer symptoms are not apparent in the early stages of the disease. Prostate cancer that is more advanced may cause signs and symptoms such as decreased force in the stream of urine, blood in the semen, discomfort in the pelvic area, bone pain, and erectile dysfunction. However, such urinary symptoms are often similar in benign prostatic hyperplasia, and these signs and symptoms cannot reliably differentiate benign prostatic disease from cancer. Prostate-specific antigen is a well-established tumor marker that aids in the diagnosis, staging, and follow-up of prostate cancer. Biochemically, PSA is a serine protease, also known as human kallikrein 3 (hK3). The majority of PSA produced by the prostate is excreted in the semen, but a small proportion “leaks” into the systemic circulation and can be measured as serum PSA. Studies by Stamey et al showed that, on a weight for weight basis, prostate cancer tissue released 30 times more PSA into the circulation than normal prostate tissue, perhaps because of loss of normal tissue architecture. 19 Although PSA has been employed for screening for the early detection of prostate cancer, its use remains controversial. Randomized data shows that PSA screening results in diagnosis at earlier stages, improved oncologic outcomes after treatment, and lower prostate cancer mortality. However, important shortcomings of PSA screening include unnecessary biopsies due to false-positive PSA tests, overdiagnosis of some clinically insignificant cancers, and potential side effects from prostate biopsy and/or prostate cancer treatment. 20 Autopsy studies have demonstrated a high prevalence of asymptomatic localized prostate cancer among men who die from other causes. This observation has led to the criticism that prostate cancer screening leads to unnecessarily aggressive intervention in many men who would not develop symptomatic disease within their lifetimes. The prevalence of prostate cancer has been reported to be 0.5%, 23%, 35%, and 46% among men in the < 50, 50 to 59, 60 to 69, and ≥70 age groups, respectively, with the majority being low-risk disease. 21 One randomized prospective trial of PSA screening for prostate cancer showed a relative reduction in mortality from prostate cancer of 21% at 13 years of follow-up. However, a total of 781 men needed to be invited to screening and 27 to be diagnosed with prostate cancer to avert 1 death from the disease, highlighting the concept that not all cancers have lethal potential within a man’s natural longevity. Despite showing a clear prostate cancer mortality reduction, these findings may not be sufficient to justify population-based screening. This ongoing controversy is highlighted by the divergent recommendations on screening from multiple professional organizations ( ▶ Table 1.1). Guideline group Recommendation (2012–2014) Year Reference Unites States Preventative Services Task Force Against 2012 Moyer et al Annuals of Int Med 2012 24 Melbourne (expert panel) For patients in good health, baseline PSA at 40–50 PSA screening as part of a multivariable approach 2013 Murphy et al BJU Int 2014 78 European Association of Urology Baseline PSA at 40–45 years 2013 Heidenreich et al Eur Urol 2014 79 National Comprehensive Cancer Network Baseline PSA at 45 years 2014 Carroll et al J Natl Cancer Inst 2014 23 American Cancer Society Shared decision making in patients ≥ 50 years 2014 Smith et al CA Cancer J Clin 2014 80 American College of Physicians Shared decision making in patients 50–69 years 2013 Qaseem et al Ann Intern Med 2013 81 European Society of Medical Oncology (expert opinion) PSA screening suitable for well-informed men at 50–75 years 2012 Horwich et al Ann Onc 2013 82 American Urological Association Shared decision making in patients 55–69 years 2013 Carter et al J Urol 2013 22 American Society of Clinical Oncology Shared decision making in patients with >10 years life expectancy 2012 Basch et al J Clin Oncol 2012 83 Abbreviations: PSA, prostate-specific antigen. The American Urological Association (AUA) guidelines from 2013 recommend individualized decisions about screening for higher risk men aged < 55 years, such as those with a positive family history and African-American men, while the National Comprehensive Cancer Network (NCCN) recommends starting the risks and benefits discussion of PSA screening at age 45. 22, 23 For men ages 55 to 69, the AUA recommends shared decision making about screening. Although this group has the strongest evidence for screening benefit, there remains potential for harm. For this reason, the AUA emphasizes the importance of a bilateral discussion about screening between the patient and physician that incorporates the benefits, risks, uncertainties, and patient’s values and preferences. Finally, the AUA recommends against routine PSA screening in men of age> 70 years, while acknowledging that some men of age> 70 years in excellent health may still benefit from screening. In comparison, the United States Preventative Services Task Force (USPSTF) recommendations from 2012 advised against routine PSA screening, given concerns related to uncertain mortality reduction, overdetection of indolent disease, as well as costs. 24 Various PSA metrics in addition to total PSA, such as PSA velocity, PSA density, free PSA, and PSA doubling time, have been incorporated into PSA screening to improve prostate cancer screening sensitivity and specificity. The rate of change in PSA over time (PSA velocity [PSAV]) is associated with both prostate cancer risk and aggressiveness. PSA velocity may increase the specificity of screening for clinically significant prostate cancer, but its ability to increase the predictive value beyond that of PSA alone is controversial. 25 PSA density (PSAD), defined as the PSA level divided by prostate volume, at the time of diagnosis has been shown to be a significant predictor of progression to treatment, along with age and PSA slope. 26 PSAD may be useful in the selection of potential candidates for active surveillance and for monitoring for subsequent disease progression in men being managed by active surveillance. In addition, PSA circulates through the blood in two ways: either bound to other proteins or unbound, referred to as free PSA (fPSA). The fPSA test measures the percentage of unbound PSA, whereas the conventional PSA test measures the total of both free and bound PSA. In men with prostate cancer, there appears to be a lower proportion of free PSA, expressed as a decrease in the ratio of free to total (f/t) PSA. In a study using an f/t PSA ratio of > 25% to determine the need for a prostate biopsy, 95% of cancers were detected and 20% of unnecessary biopsies avoided. 27 These findings resulted in the Food and Drug Administration approving the f/t PSA ratio for helping guide decisions to perform a biopsy in men with a PSA of 4 to 10 ng/mL. However, the free fraction is the most labile with rapid degradation even at 4 degrees Celsius, which may impact the utility of the ratio in clinical practice. Finally, the PSA doubling time, defined as the time required for the PSA level to double, has been applied as an indicator of clinical progression in patients with prostate cancer. 28 For example, a short PSA doubling time (< 3 months) appears to be significantly associated with the onset of recurrence and the risk of death from prostate cancer. A longer PSA doubling time is associated with a longer time to metastasis, to prostate cancer–specific death, and to death from all causes. 29 In addition to these conventional PSA derivatives to enhance PSA-based prostate cancer screening, a generation of prostate cancer biomarkers has emerged, consisting of serum-, urine-, and tissue-based assays that may supplement PSA testing. These biomarkers are being explored for various purposes, including (1) to help clinicians determine whom to biopsy, such as PCA3, 30 the Prostate Health Index (phi), 31 and 4K score 30 (with the latter two representing advanced ratios based on PSA isoforms); (2) to help clinicians determine when to perform a repeat biopsy following a prior negative biopsy, such as ConfirmMDx, 32 Prostate Core Mitomic Test, TMPRSS2-ERG, 32 and the phosphatase and tensin homolog (PTEN) gene; 32 (3) to help clinicians determine which men with a positive biopsy to treat or not treat, such as Oncotype DX 32 and Prolaris® 32 and (4) to help clinicians predict the probability of metastasis after radical prostatetomy, such as Decipher. 32 Although these biomarkers hold promise for assisting clinicians in improving risk assessment, reducing overtreatment, and providing more selective therapy for patients with high-risk disease, further understanding is needed to appreciate their potential benefits and limitations. Additional outcomes data and standardization in clinical usage are ultimately needed to guide practice. Biopsy of the prostate to diagnose or exclude cancer is performed nearly 1 million times annually in the United States most frequently as a result of an elevated PSA. 33 Most biopsies are conducted under ultrasound guidance by a transrectal approach ( ▶ Fig. 1.1). Using this technique, tissue cores are obtained systematically throughout the prostate, most commonly using a 12-core biopsy template, matching the approach supported by the American Urological Association ( ▶ Fig. 1.2). 34 A variety of techniques have emerged for optimizing this biopsy scheme, including computerized and image-guided techniques, although conventional systematic sampling employing a variable number of cores remains the standard in practice. The use of a 12-core systematic biopsy that incorporates apical and lateral cores of the prostate increases cancer detection rates compared to traditional sextant sampling methods, reduces the likelihood that patients will require a repeat biopsy given improved negative predictive value, ultimately allows more accurate risk stratification, and does not appear to increase the likelihood of detecting insignificant cancers as compared to the 6-core sextant sampling. 34, 35 However, only limited evidence supports the use of initial biopsy schemes involving more than 12 cores. Fig. 1.1 Schematic of standard transrectal ultrasound guided prostate biopsy being performed. Fig. 1.2 12-core extended systematic prostate biopsy template with lateral cores. LBL, left base lateral; LBM, left base medial; RBM, right base medial; RBL, right base lateral; LML, left mid lateral; LMM, left mid medial; RMM, right mid medial; RML, right mid lateral; LAL, left apex lateral; LAM, left apex medial; RAM, right apex medial; RAL, right apex lateral. Currently, both end fire and side fire configurations of the biopsy probe are used for prostate sampling ( ▶ Fig. 1.3). While endfire and sidefire ultrasound probes are generally viewed as having similar cancer detection rates and complications, and are thus both used in clinical practice, some recent literature suggests slightly higher detection rates with an endfire probe. 36, 37 The side fire transrectal probe has been associated with a better patient tolerance profile. 37, 38 Fig. 1.3 (a) Endfire and (b) sidefire configurations of the biopsy probe used for prostate sampling. Improvements in anesthesia techniques have allowed urologists to obtain a greater number of cores as well as cores from different locations in the gland, such that urologists may potentially perform a “saturation” biopsy procedure within an office setting that incorporates a very large number of transrectal cores. 39 A periprostatic nerve block is commonly used during transrectal ultrasound (TRUS) guided biopsy in which the optimal injection site seems to be the angle between the prostate and the seminal vesicles, which can be easily identified as a hypoechoic area on TRUS. A concentration of 1% lidocaine (5 mL per side) is sufficient to provide pain relief. Despite lack of a standardized dose or optimal technique, periprostatic nerve block remains the clinical gold standard. 40 According to the AUA clinical guidelines on the incidence, prevention, and complications related to prostate needle biopsy, the most common urological side effects of a prostate needle biopsy include hematuria, rectal bleeding, hematospermia, urinary tract infection, and acute urinary retention. 41, 42 Erectile dysfunction and vasovagal response also may occur following prostate biopsy, although they are generally self-limiting and well tolerated ( ▶ Table 1.2). Most infectious complications after prostate biopsy are limited to symptomatic urinary tract infection and low-grade febrile illness, which can be readily treated with oral or intravenous antibiotics. However, postbiopsy sepsis has also emerged as a risk of this procedure. The incidence of infectious complications following prostate biopsy in large multi-institutional studies ranges from 0.1 to 7% depending upon the antimicrobial prophylactic regimen used, 43, 44, 45 with approximately 30 to 50% of these patients having accompanying bacteremia. 46, 47 In a meta-analysis and review of prostate biopsy results, Shen et al determined that there were no significant differences in the incidence of major or minor complications between the transperineal and transrectal technique. 48 However, the nature of the complications is different, with infection being more common with the transrectal route. Complication Incidence Hematuria Rectal bleeding Hematospermia Urinary tract infection 2–6% 88 Bacteremia 0.1–2.2% 46 Hospitalization 0.6–4.1% 44 Erectile dysfunction 2.2% 89 Urinary retention Vasovagal response Current considerations for the prevention of bleeding complications after prostate biopsy include withholding anticoagulants, including warfarin, nonsteroidal anti-inflammatory drugs, herbal supplements, and clopidogrel, for 7 to 10 days prior to the biopsy when it is possible to do so. Fluoroquinolones or cephalosporins remain the recommended prophylactic antibiotics, although the frequency of quinolone-resistant infections is increasing. Prebiopsy screening with rectal swabs may allow identification of those men harboring antibiotic-resistant organisms in their endogenous gastrointestinal flora and for whom fluoroquinolone prophylaxis may not be appropriate. Prebiopsy rectal swabs have identified fluoroquinolone-resistant isolates in up to 22% of patients and are considered to have contributed to the decrease in postbiopsy infection. 43, 49 The contemporary random 12-core systematic biopsy strategy relies on sampling efficiency for cancer detection and is consequently subject to sampling error ( ▶ Fig. 1.4). 50 Cancers are often small, intermingled with benign stroma, and not uniformly distributed within the gland. As a result, clinically significant cancers frequently go undetected. Undersampling of the prostate during ultrasound-guided biopsy also leads to incorrect risk stratification in a subset of men, given the potential to erroneously categorize clinically significant tumors as low volume or low grade. Random non targeted prostate biopsies risk inadequate sampling of a cancer lesion, often at its periphery. For instance, the biopsy may only demonstrate a small length of the tumor having a low Gleason score, when in fact, a clinically significant portion with a higher Gleason score may exist adjacent to the site of the positive biopsy core. Approximately 30 to 50% of men over age 50 years harbor clinically insignificant prostate cancer at autopsy. These clinically insignificant cancers are often identified by chance during a systematic biopsy approach, contributing, in part, to the problem of overdetection and overtreatment of indolent prostate cancer. Repeat biopsies in patients with persistent clinical suspicion for prostate cancer serves to further increase detection of clinically insignificant prostate cancer. The recent trend of trying to overcome sampling error by increasing the number of cores obtained during a single biopsy session, or by repeated biopsy sessions, further escalates the risk of identifying small, indolent cancers, which may have little relationship with the patient’s PSA elevation, and also escalates the overall costs. 34 Fig. 1.4 Current limitations of prostate biopsy. (a) Clinically insignificant cancers are often identified by chance during a systematic biopsy (oversampling). (b) Systematic biopsies may lead to incorrect risk stratification categorizing clinically significant tumors as low volume or low grade (undersampling). (c) Systematic deployment of needle biopsies may lead to clinically significant tumors being missed on initial biopsy (undersampling).
1.2 Public Health Burden
1.3 Risk Factors
1.4 Symptoms
1.5 Prostate-Specific–Antigen Screening for the Early Detection of Prostate Cancer
1.5.1 PSA Derivatives and Emerging Prostate Cancer Biomarkers
1.6 Systematic Transrectal Ultrasound-Guided Prostate Biopsy
1.6.1 Complications of Prostate Biopsy
1.6.2 Prevention of Prostate Biopsy Complications
1.7 Limitations of Contemporary Systematic Biopsy Technique
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree