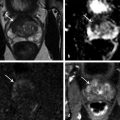
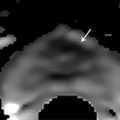
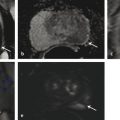
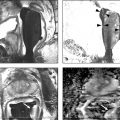
Prebiopsy Planning The large majority of prostate cancer cases are multifocal, comprising numerous tumor foci within the gland. Nevertheless, prostate cancer may be regarded as a biologically unifocal disease, in which a single most aggressive tumor focus, termed the index lesion, drives the natural course of the disease and any adverse oncologic outcome by being the origin of any distant metastases. 8, 9, 10 Therefore, a key goal of prostate MRI in the prebiopsy setting is to define and localize an index lesion, which can then be biopsied in order to best characterize the lesion and guide subsequent management. Prebiopsy MRI is generally performed in three clinical contexts. 11, 12, 13, 14, 15 These are patients with clinically suspected prostate cancer usually based on an elevated or increasing serum prostate specific antigen (PSA) level (PSA > 4.0 ng/mL), an increasing PSA velocity, or an abnormal digital rectal examination, who only have a prior negative biopsy, typically performed as a nontargeted transrectal ultrasound–guided (TRUS-guided) biopsy using standard systematic sampling. MRI can detect prostate cancer in over 50% of men who have undergone a prior negative biopsy. 6, 16, 17, 18 These are patients with clinically suspected prostate cancer who have not undergone a prior biopsy. This represents a growing group of patients and would represent the majority of patients undergoing MRI in the future if performing MRI in biopsy-naïve patients were to become the clinical standard of care. An advantage of performing MRI before any biopsy is performed is avoidance of any negative impact on interpretation from postbiopsy hemorrhage. An additional potential advantage, although controversial, is that if the MRI shows no suspicious lesion, then biopsy may potentially be avoided altogether. 19 Prostate MRI in biopsy-naïve patients may be covered by some insurance carriers, given that an elevated PSA may be viewed as a presumptive diagnosis of prostate cancer. These are patients with biopsy-proven low-grade cancer who elect to defer immediate definitive treatment after careful consultation with prostate specialists. Although variable criteria are applied clinically for considering a patient with prostate cancer to be a candidate for active surveillance, traditional strict criteria require low-risk disease as defined by the Epstein criteria (Gleason Score (GS) < 7 at biopsy, < 3 positive biopsy cores, < 50% of all biopsy cores positive, and a serum PSA <10 ng/mL). 20 Active surveillance patients require close follow-up and potential intervention at a future time in the event of any evidence of disease progression. Traditionally, active surveillance regimens incorporate an early repeat biopsy to confirm the presence of low-risk disease, followed by additional repeat biopsies every 1 to 3 years for at least 10 years or until life expectancy is less than 10 years. However, prebiopsy and MRI-targeted biopsy has great potential to reduce the frequency of repeat biopsies for men being managed by active surveillance. State-of-the-art prostate MRI technique has been discussed extensively in prior chapters. Given potentially large volumes of patients undergoing prostate MRI in the prebiopsy setting, protocols need to be efficient and provide the necessary information for biopsy planning without extraneous imaging that would decrease patient throughput. Thus, the technique is optimized for cancer detection and localization, not for locoregional staging. 21 To screen patients for prostate MRI, practitioners should: Screen patients for MR eligibility (for example, MR-incompatible hardware is a contraindication). Screen patients for recent prostate therapy. MRI should be performed at least 3 weeks, ideally 6 to 8 weeks, after a prior prostate biopsy or treatment (surgery, radiation, chemotherapy). Ideal patient preparation is simple and cost effective. Ensure complete evacuation of the rectum prior to scanning via use of an enema or suppository- and even rectal gas aspiration using a flexible tube. Some practices also administer an antiperistaltic agent (i.e., glucagon [unless there is a history of poorly controlled diabetes mellitus], scopolamine butylbromide), or hyoscine butylbromine] although there is a paucity of supporting data, for use of such an agent. Technical parameters and recommendations for acquiring multiparametric MRI of the prostate are described in detail in Prostate Imaging–Reporting and Data System version 2 (PI-RADS v2). 22 While a 1.5-T system may be used, a 3-T MR system is optimal. Nonetheless, while the signal-to-noise ratio (SNR) is better at the higher magnetic field strength, some artifacts may also be worse, thus one must be familiar with one’s system and technical optimization regardless of field strength. A multichannel (at least 8 channels) external phased-array pelvic coil is suggested for use in the prebiopsy setting, in which MRI is being performed to detect and localize dominant lesions for targeted biopsy. The reduced cost and examination time of this approach, in comparison with use of an endorectal coil (ERC), is an important consideration when applying prostate MRI in large patient populations in prebiopsy settings. In addition, this approach avoids the potential susceptibility artifact and geometrical distortion that may result from use of an ERC. Furthermore, use of an external coil may increase the willingness of patients not facing imminent therapy for prostate cancer to undergo an MRI examination. Nonetheless, an ERC may be considered in patients with a large body habitus, in which case SNR at the prostate is relatively limited, or in patients for whom surgery is considered likely and a more detailed characterization of the posterior prostate capsule and neurovascular bundles is desired. The image sequences performed 23, 24, 25 must be reliable yet efficient in detecting suspicious lesions for targeted biopsy. Detailed characterization and staging, if necessary, may be performed at the time of a later examination. High matrix, small field-of-view acquisitions are preferred. Axial T2-weighted images (T2WI) are the workhorse of anatomical prostate assessment, providing high spatial resolution and delineating zonal anatomy, as well as locating various benign conditions such as prostatic hyperplasia and prostate cysts. Prostate cancer is generally T2 hypointense, although the specificity of T2WI for cancer is usually limited given the overlapping appearance on T2WI of various benign and malignant conditions. The differential diagnosis of a T2-hypointense lesion includes: cancer, hemorrhage (e.g. postbiopsy or trauma), benign prostatic hyperplasia, post–radiation therapy changes, post–hormonal therapy changes, and postinfectious or postinflammatory fibrosis. Furthermore, T2WI does not predict tumor aggressiveness. Diffusion-weighted imaging (DWI) provides physiologic information. As described in prior chapters, it offers microarchitectural tissue characterization based on the brownian motion of water molecules. Prostate cancer cells are more densely packed than healthy cells, leading to increased restriction of water movement. Therefore, DWI can distinguish malignant from benign lesions as well as low-grade from high-grade tumors, thereby providing increased specificity in cancer detection. 26 T1-weighted dynamic contrast enhanced (DCE) imaging provides further physiologic information through microvascular tissue characterization based on the distribution of an intravenously administered gadolinium-containing contrast agent. Due to neovascularization, malignant lesions are frequently hypervascular. The precontrast images from the DCE acquisition can be used for the detection and localization of hemorrhage without requiring a separate dedicated T1-weighted acquisition for this purpose. Magnetic resonance spectroscopic imaging (MRSI) is usually deferred, given practical considerations of availability, variable technical expertise, general requirement for an endorectal coil, and the at least 10 minutes additional scanner time needed for acquisition. Large field-of-view images for locoregional evaluation may also be deferred in the prebiopsy setting. Though multiple systems have been suggested, standardized reporting is recommended using PI-RADS v2, which facilitates communication between radiologists as well as with referring physicians, including both urologists and primary care physicians. 22 PI-RADS v2 was developed as a joint effort of the American College of Radiology (ACR), the European Society of Urogenital Radiology (ESUR), and AdMeTech Corporation. PI-RADS v2 employs standardized language based on RadLex, which is a comprehensive lexicon of well-defined radiology terms developed by the Radiologic Society of North America (RSNA). As discussed in Chapter 6, PI-RADS v2 not only defines the vocabulary but also the technical parameters required for prostate MRI and enhances research and quality assurance. A critical advantage of using PI-RADS v2 is the assignment of discrete assessment categories to lesions to summarize the level of suspicion for aggressive prostate cancer. Though these assessment categories do not specify management, general guidelines for management (following the arrows in the list below) can be associated with them: Very low to low suspicion (PI-RADS v2 categories 1 and 2) → defer targeted biopsy given the low likelihood of the target representing significant cancer; although further data remains required, deferral of standard systematic biopsy may be considered as well; continue monitoring as clinically warranted Moderate suspicion (PI-RADS v2 category 3) → consider biopsy versus follow-up; further data regarding outcomes of PI-RADS category 3 lesions remains required, and there is currently a lack of consensus regarding management of such lesions High suspicion (PI-RADS v2 category 4) → targeted biopsy Very high suspicion (PI-RADS v2 category 5) → targeted biopsy; consider repeat targeted biopsy if the initial targeted biopsy is pathologically negative An additional aspect of interpreting prostate MRI for targeted biopsy planning is three-dimensional (3D) segmentation of the prostate gland and any identified biopsy targets. Although unnecessary prior to in-bore biopsy, the segmentation is crucial in advance of image-fusion–targeted biopsy. The segmentation may be deferred at the time of initial MRI reporting, and then performed at a later date once the targeted biopsy is scheduled. Software packages used for segmentation may also be used to generate components of the report, including automatic capture of salient images. The goal of prostate biopsy is essentially twofold 27: (1) to establish the presence or absence of cancer and (2) to determine the tumor grade as defined by the Gleason score (GS). A variety of methods to perform a prostate biopsy are available, ( ▶ Table 9.1) which in this section are categorized by modality, approach, and strategy. 28 The imaging modalities that can be used in targeted prostate biopsy are MRI, ultrasound (US), and computed tomography (CT). MRI and ultrasound are most common and can be used alone or in MRI-US fusion techniques. CT is used in the rare cases in which MRI and ultrasound are not feasible. The transrectal approach is generally considered the standard of care as of 2016. This is typically performed using local anesthesia delivered to the periprostatic nerve plexus. The transperineal or transgluteal approaches are acceptable if the transrectal approach is not feasible (e.g., due to prior anorectal resection). Although controversial, such approaches are preferred by some practitioners due to a potential decreased risk of sepsis. A relative disadvantage of this approach is the requirement for conscious sedation. Systematic biopsy entails methodically sampling the entire prostate according to a gridlike map in order to maximize the chance of detecting significant prostate cancer. No single area of the prostate is specifically targeted. In routine clinical practice, 12 cores are obtained (medial and lateral cores from each of six sextants: right and left lobes at the levels of the base, midgland, and apex). However, a saturation biopsy comprising a larger number of cores (i.e., 30, if not more, cores) may be performed in an effort to further increase the cancer detection rate. ( ▶ Fig. 9.1a). The advantages include that systematic biopsy is simple, is widely available, urologists are familiar with it and are trained in the procedure, and it does not require the additional expense of advanced technology for performing targeted biopsy. Systematic biopsy is supported by current American Urological Association (AUA) guidelines and represents the standard of care in most communities. The disadvantages of systematic biopsy include overdetection of insignificant lesions and underdetection or undergrading of significant lesions due to undersampling (particularly of the anterior and paramedian aspects of the prostate), all of which result in incorrect risk stratification and possible need for repeat biopsies due to uncertainty with a false-negative rate up to 47%. 29 In addition a greater number of cores are obtained in a single biopsy session in comparison with an approach in which only targeted cores are obtained. Targeted biopsy entails directed sampling of lesions detected by mpMRI ( ▶ Fig. 9.1b). 30 Typically, the patient first undergoes mpMRI to identify the index lesion and any secondary targets. Then, during a subsequent session, targeted biopsy is accomplished in one of two ways: Direct (in-bore) biopsy, in which limited MRI sequences are performed to localize the previously identified abnormalities and provide needle guidance Indirect (fusion) biopsy, in which the MR images are fused to ultrasound images to be used for biopsy guidance. Fusion can be performed with or without software, as will be discussed in 9.3.4 MRI-Transectal Ultrasound–Fusion Biopsy. In comparison with systematic biopsy, targeted biopsy improves detection of significant cancers (30% more high-risk cancers detected), reduces overdetection of insignificant cancers (17% fewer low-risk cancers detected), 31, 32 and better predicts the GS from radical prostatectomy (GS concordance of 81% for MRI/US fusion biopsy vs. 40–65% for conventional systematic TRUS biopsy). 33 Furthermore, repeat biopsy of an imaging-defined target may allow for more reliable follow-up of prostate cancer patients undergoing active surveillance than is achieved by serial systematic biopsy. One European study suggested that over a 10-year period following initial clinical suspicion for prostate cancer, targeted biopsy achieves similar costs but improved quality of life compared with systematic biopsy, given the reduction in overdiagnosis and overtreatment from improved risk stratification. 34 The disadvantages of targeted biospy are: it is more complex and resource intensive; urologists have less experience doing it than systematic biopsy; it has a potentially longer procedural time than systematic biopsy; it is offered by a limited number of centers at present; there are high costs for purchasing technology for targeting; there is a lack of available data on targeted biopsy in comparison with systematic biopsy at present; and the time needed for utilization of MRI gantry (for the in-bore approach only). Fig. 9.1 Coronal views of the prostate comparing systematic and targeted biopsy approaches. (a) Anatomical distribution of cores for conventional systematic biopsy. Although typically entailing 12 cores, saturation biopsies may be performed, entailing a much larger number of cores (i.e., 36 cores in this illustration). (b) In comparison, targeted biopsies may obtain as few as 3 to 4 samples from a single imaging-identified target that is considered to be the most likely site of any clinically significant tumor that may be present. (CZ, central zone; PZ, peripheral zone; TZ, transition zone.) Given the diversity of modalities, approaches, and strategies, there are many different scenarios available for performing prostate biopsy. However, existing data suggests that targeted biopsy provides overall optimal results when considering both detection of significant prostate cancer and reducing overdetection of insignificant cancer. For example, one prospective study of 1,003 men undergoing both MRI-US fusion–targeted biopsy and systematic biopsy during the same biopsy session concluded that systematic biopsy would need to be performed in addition to targeted biopsy in 200 men in order to diagnose 1 additional high-risk cancer, and for each such additional high-risk cancer identified, 17 additional low-risk cancers would be diagnosed. 31 In addition, a meta-analysis of 14 studies of indirect MRI-US targeted biopsy (with or without software-based image fusion) concluded that targeted biopsy detects more significant cancers using substantially fewer biopsy cores than does systematic biopsy alone. 35 Nonetheless, achieving optimal outcomes from targeted prostate biopsy requires high-quality MR image acquisition, image interpretation, and targeting technique. Errors in any of these aspects will diminish the performance of MRI-targeted biopsy in clinical practice in comparison with the high performance anticipated based upon the published literature. As of 2016, the most common approach to performing prostate biopsy is systematic TRUS-guided biopsy, which is considered the standard of care for most communities. However, multiple investigations have shown that it is a suboptimal technique on its own. 32, 35 Detection rates are at least 40% on first biopsy, 20% on second biopsy, and 10% on third biopsy. 36, 37 Sensitivity is limited, reported to be 62% in the base, 52% in the midgland, and 38% in the apex. 38 The poor sensitivity in part reflects the typically inconspicuous appearance of tumors within the prostate by ultrasound, 38 as tumors may be isoechoic or unable to be discriminated from benign nodules. 12 Because targeted biopsy based on ultrasound alone is generally not feasible, the systematic approach is overwhelmingly favored, despite incomplete sampling. The false-negative rate varies, although it is estimated to be up to 47%. 39, 40 Detection rates using ultrasound alone are improved through the use of color power Doppler and intravenous ultrasound contrast. 41 Although requiring further investigation, software systems for performing fusion-based targeted biopsy may also offer the ability to optimally space the nontargeted systematic cores across the prostate, thereby potentially improving the detection rate that is achieved by systematic biopsy. PATHOLOGY REPORT SPECIMEN(S): A. RIGHT BASE/MIDGLAND PERIPHERAL ZONE X 6 CLINICAL INFORMATION: 73 yo M with three negative prior biopsies. Elevated PSA. FINAL DIAGNOSIS: A1. PROSTATE, RIGHT BASE PERIPHERAL ZONE/MIDGLAND (BIOPSY): Prostatic adenocarcinoma, Gleason grade 4+4 = 8/10, involving multiple fragmented cores, measuring 8 mm, occupying 70% of biopsy tissue No perineural invasion A2. PROSTATE, RIGHT BASE PERIPHERAL ZONE/MIDGLAND (BIOPSY): Prostatic adenocarcinoma, Gleason grade 4+4 = 8/10, involving fragmented cores, measuring 1 mm, occupying 20% of biopsy tissue No perineural invasion A3. PROSTATE, RIGHT BASE PERIPHERAL ZONE/MIDGLAND (BIOPSY): Prostatic adenocarcinoma, Gleason grade 4+4 = 8/10, involving multiple fragmented cores, measuring 5 mm, occupying 60% of biopsy tissue No perineural invasion A4. PROSTATE, RIGHT BASE PERIPHERAL ZONE/MIDGLAND (BIOPSY): Prostatic adenocarcinoma, Gleason grade 4+4 = 8/10, involving 2 of 2 cores, measuring 8 mm discontinuously, occupying 70% of biopsy tissue No perineural invasion A5. PROSTATE, RIGHT BASE PERIPHERAL ZONE/MIDGLAND (BIOPSY): Prostatic adenocarcinoma, Gleason grade 4+4 = 8/10, involving fragmented cores, measuring 4 mm, occupying 50% of biopsy tissue No perineural invasion A6. PROSTATE, RIGHT BASE PERIPHERAL ZONE/MIDGLAND (BIOPSY): Prostatic adenocarcinoma, Gleason grade 4+4 = 8/10, involving 1 of 2 cores, measuring 1.5 mm, occupying 15% of biopsy tissue No perineural invasion Fig. 9.2 (a) Radiology suite with a 1.5-T MRI magnet used for direct in-bore targeted prostate biopsy. All objects inside the room during the procedure must be MRI compatible, including the biopsy instrument and potential surgical hardware inside the patient’s body. (b) Invivo DynaTrim Automated Biopsy Gun (top; both 150-mm and 170-mm needle lengths available) and Needle Guide (bottom). The biopsy procedure is semisterile. Peri-procedural antibiotic prophylaxis is provided to the patient. (c) Invivo DynaTrim Biopsy Device. The biopsy device provides needle control through three controllers. (d) The location of the needle-guide holder is described in a three-dimensional polar coordinate system. Controller #1 adjusts the horizontal angle (degrees). Controller #2 adjusts the vertical angle (degrees). Controller #3 adjusts the z-axis distance (mm) that the needle-guide holder is advanced along its track. (e) Invivo DynaTrim Computer Console. The polar coordinates to which the biopsy device shown in (e) is adjusted are derived by the computer through the calibration process and displayed on the computer screen. (f) Invivo DynaTrim Biopsy Device in place. With the patient positioned prone on the MRI gantry table, the biopsy device is placed in between the lower extremities and the needle guide is positioned in the rectum.
9.2.1 Target Population: Who Needs a Prebiopsy MRI?
Biopsy-Negative Patients
Biopsy-Naïve Patients
Active Surveillance Patients
9.2.2 Prebiopsy MRI Technique
Patient Screening and Preparation
Equipment
Field Strength
Coils
Image Sequences
T2-Weighted Imaging
Diffusion-Weighted Imaging
Dynamic Contrast-Enhanced Imaging
MR Spectroscopic Imaging and Large Field-of-View Images
9.2.3 MRI Results and Reporting
9.3 Biopsy Guidance
9.3.1 Biopsy Options
Modality
Approach
Systematic Biopsy
Technique
Advantages
Disadvantages
Targeted Biopsy
Technique
Advantages
Disadvantages

Summary
9.3.2 Transrectal Ultrasound–Guided Biopsy
9.3.3 Direct Transrectal MRI-Guided Biopsy (also Known as In-Bore or MRI Fusion Biopsy)

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree