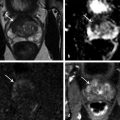
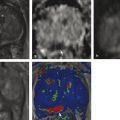
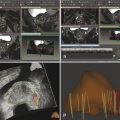
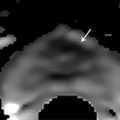
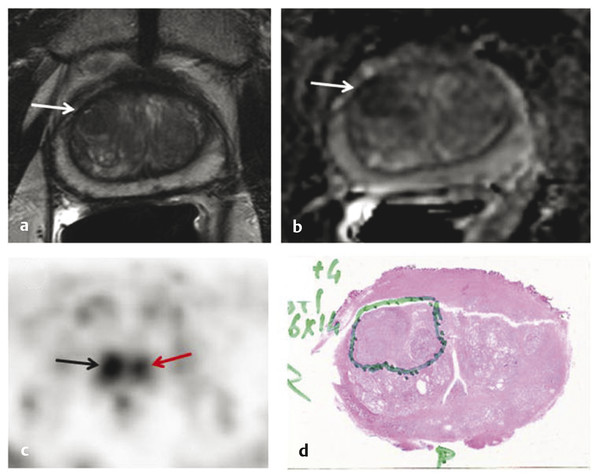
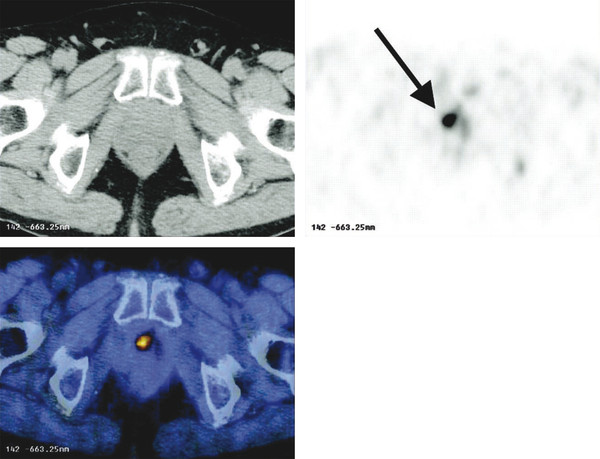
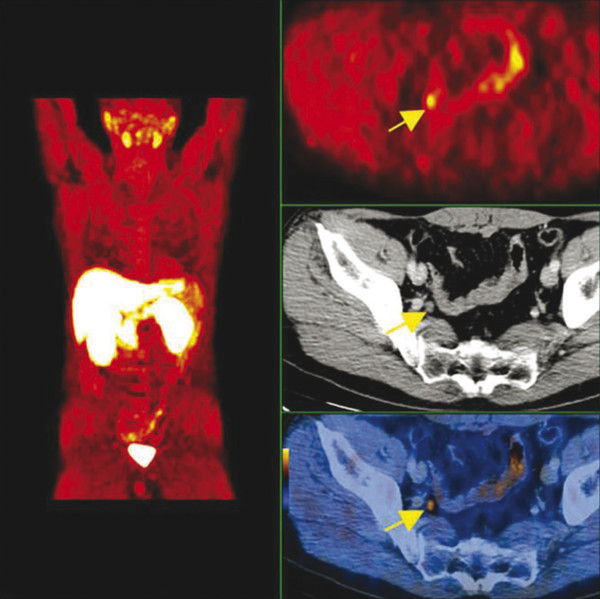
Summary of select PET radiotracers employed in prostate cancer Radiotracer Biological basis Major potential utility in prostate cancer 18F-fluorodeoxyglucose Glucose metabolism Detection of aggressive primary tumor (Gleason score>7) Assessment of therapy response and prognostication in mCRPC 11C-acetate Lipogenesis BCR 11C-choline Lipogenesis BCR 18F-fluorocholine Lipogenesis BCR 18F-NaF Bone surface hydroxyapatite matrix Bone metastasis 18F-FMAU Cellular proliferation (thymidine analog) Primary tumor characterization (investigational) anti-18F-FACBC Amino acid metabolism BCR Radiolabeled PSMA targeted agent (e.g. 68Ga-PSMA) Prostate-specific membrane antigen (external moiety) Primary tumor detection/localization (investigational) BCR (investigational) Abbreviations: mCRPC, metastatic castration-resistant prostate cancer; BCR, biochemical recurrence; 18F-FMAU, 2′-deoxy-2′-[18F]fluoro-5-methyl-1-β-D-arabinofuranosyluracil; anti-18F-FACBC, anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid; PSMA, prostate specific membrane antigen; 68Ga-PSMA, Glu-NH-CO-NHLys-(Ahx)-[68Ga-HBED-CC] conjugate which binds the motif glutamate-urea-lysine with the chelator HBED-CC. Prostate cancer is typically considered as a suspected diagnosis after an abnormal digital rectal examination and/or a high or rising serum prostate-specific antigen (PSA) level. The usual diagnostic approach includes standard 10- to 12-core TRUS-guided biopsy. However, the miss rate with TRUS-guided biopsy may be as high as 40% and even higher (up to 70%) in repeat biopsies. 4, 5 TRUS-guided biopsy generally lacks sufficient sensitivity and specificity to detect and localize prostate cancer, although ancillary techniques such as elastography and contrast enhancement may be potentially useful. 6 Image-guided biopsy optimizes the probability of detection of clinically relevant tumors (e.g., aggressive tumors) and reduces the biopsy rate of clinically indolent tumors. Image-guided tumor localization and characterization allows for better informed treatment decision making, including selecting patients with low-grade tumors for active surveillance and selecting some patients with higher-grade tumors for focal therapy. Multiparametric MRI including DWI and DCE-MRI at 3 T using pelvic phased-array and endorectal coils has offered improved diagnostic performance for the imaging evaluation of the prostate gland. 7, 8 Additionally, some investigators have included magnetic resonance spectroscopy imaging (MRSI) as a component of mpMRI, although this procedure is not routinely employed clinically in view of the need for specialized expertise in its execution and interpretation, longer imaging time, and, probably more importantly, the lack of definite evidence for significant incremental diagnostic contribution to those derived from the other in multiparametric MRI. 9 Prostate cancer is typically characterized by low T2 signal intensity replacing the normally high T2 signal intensity in the peripheral zone. However, this feature has limited sensitivity since some tumors are isointense. 7 Specificity is also limited since hemorrhage, scar, prostatitis, atrophy, and post-treatment changes may also result in low T2 signal intensity. Diffusion-weighted imaging measures the brownian motion of free water molecules within the tissue. Prostate cancer generally demonstrates reduced diffusion of water, which has been attributed to the increased cellularity of malignant tissue and the reduction of the extracellular space. 10 The measured parameter that reflects water diffusion is the apparent diffusion coefficient (ADC), which is typically 20 to 40% lower in malignant lesions than in benign lesions or normal prostatic tissue. 7 DCE-MRI employs kinetic modeling typically with the external iliac artery serving as the arterial input function and a transfer constant Ktrans that describes the microvascular permeability and blood flow. 11 Prostate cancer demonstrates increased tumor vascularity manifested as early, rapid, and intense hyperenhancement followed by rapid washout of contrast material from the tumor in comparison to normal prostate tissue. 12 DCE-MRI may be able to differentiate high-grade prostate cancer from chronic prostatitis, although low tumor volumes and infiltrative prostate cancer may be missed. 13 A meta-analysis published in 2014 of the accuracy of mpMRI reported relatively high specificity but variable sensitivity for prostate cancer detection. 14 While another systematic review and meta-analysis reported a similar finding, it interestingly also found that MRI-targeted biopsy and standard TRUS-guided biopsy did not differ significantly in overall prostate cancer detection. 15 Nevertheless, MRI is particularly helpful for differentiating between organ-confined disease (stage T1 or T2) and early extraprostatic extension or seminal vesicle invasion (stage T3). The delineation of the extent of local disease can have important ramifications on treatment selection and patient management. There is an increasing interest in the potential role of PET in prostate cancer imaging. 16 Given the remarkable biological and clinical heterogeneity of prostate cancer, PET would be an ideal imaging tool for noninvasive interrogation of the underlying tumor biology in different phases of this prevalent disease. The cumulative current experience with PET and the most studied radiotracers, namely 18F-FDG, 11C-acetate and 18F- or 11C-choline, suggests a generally limited role for these radiotracers in the imaging-based localization and characterization of primary prostate tumor due to the overlap of uptake among normal tissue, benign prostatic hyperplasia, and prostate cancer. 17 The tumor uptake of FDG is based on the Warburg effect, a cancer-induced change in metabolism characterized by an increased rate of aerobic glycolysis rather than the typical mitochondrial oxidative phosphorylation, leads to complex biological mechanisms involved in malignancy-induced enhanced glucose metabolism. 18 Shiiba et al correlated the FDG uptake level in primary prostate cancer with the biopsy specimen’s Gleason score and found that at a cutoff SUVmax of 2.8, the sensitivity and specificity for differentiating between biopsy specimens with a Gleason score of 5 or less and specimens with a Gleason score of 6 or greater were 62%, and 80%, respectively. 19 Minamimoto et al evaluated FDG PET/CT for detecting prostate cancer in 50 men with elevated serum PSA levels who underwent subsequent prostate biopsy. 20 The sensitivity and specificity were 51.9% and 75.7% for the entire prostate gland, 73% and 64% for the peripheral zone, and 22.7% and 85.9% for the transition zone, respectively. The conclusion was that FDG PET/CT might be useful for detection of peripheral zone prostate cancer in men at more than intermediate risk. A systematic review and meta-analysis published in 2014 of 47,935 men reported a pooled prevalence of 1.8% for incidental high FDG uptake in the prostate gland. 21 The pooled risk of malignancy with biopsy verification was 62% (95% confidence interval [CI]: 54–71%). In a similar investigation from South Korea that included 47,109 patients, the prevalence of incidental high FDG uptake in the prostate gland was 2.8% with the rate of observed malignancy being related to the serum PSA level (3.8% rate of cancer with PSA less than 2.5 ng/mL, but 60% rate of cancer with PSA greater than 2.5 ng/mL). 22 These studies suggest that in some cases FDG PET may be able to characterize prostate tumors of sufficient size and malignancy grade (Gleason score of 7 or higher). In summary, FDG PET/CT is typically not useful for initial staging of disease, although in selected cases with high clinical suspicion for metastatic spread, it may be useful to delineate the extent of the metabolically active disease. The results for the utility of lipogenesis radiotracers, 11C-acetate and 18F- or 11C-choline in the imaging evaluation of prostate cancer are generally similar. 23 Acetate is transported across the cellular membrane via monocarboxylate transporter and participates in production of phospholipids in the cellular membranes in a reaction catalyzed by the enzyme fatty acid synthase, which is upregulated in cancer. 24 Choline enters the cell via choline transporters and forms phosphorylcholine (in a reaction catalyzed by choline kinase , which is upregulated in cancer) which is then used to generate phosphatidylcholine in the tumor cell membrane. 25 A systematic review and meta-analysis of 11C-acetate PET/CT reported a pooled sensitivity of 75.1% (]95% CI: 69.8–79.8%) and pooled specificity of 75.8% (95% CI: 72.4–78.9%) for detection of primary prostate cancer. 26 Similar to the case with FDG, the uptake level of lipogenesis tracers in benign and neoplastic prostate tissues can overlap, which is fundamentally related to the nonspecificity of these tracers for cancer ( ▶ Fig. 11.1). 27 Fig. 11.1 A 69-year-old man with prostate cancer. Axial T2-weighted MRI (a) and apparent diffusion coefficient map (b) show focal low signal intensity in the right midanterior transition zone (white arrows), corresponding to increased 11C-acetate uptake in positron emission tomography (PET) (black arrow) (c) and tumor (Gleason score 4+4) on histopathology inked in green (d). (outlined in green; (d).). Focal 11C-acetate uptake in the left transition zone corresponds to a benign prostatic hyperplasia nodule (red arrow) (c). (Reproduced with permission of Mena et al 2012, 27 Fig. 3. Both radiolabeled acetate and radiolabeled choline may be useful for initial staging in patients with intermediate to high risk for lymph node involvement. Haseebuddin et al performed 11C-acetate PET/CT in 107 men with prostate cancer with intermediate to high risk (for lymphadenopathy either Gleason score of 7 and serum PSA level greater or equal to 10 ng/mL, or Gleason score greater than or equal to 8, or serum PSA level greater than or equal to 20 ng/mL) who were scheduled to undergo radical prostatectomy. 28 The sensitivity and specificity for detection of pelvic lymphadenopathy were 68% and 78%, respectively. Patients with positive PET scans had 3.3 times higher risk for therapy failure after surgery. Therefore, in selected intermediate- and high-risk cases, 11C-acetate may provide useful information that can lead to management change at the time of initial staging. 29 The detection of prostate tumor with 11C-choline may depend on tumor configuration, with unifocal cancers detected more often than those that are multifocal or rindlike. Moreover, the extent of actual tumor may not completely overlap the area with abnormal uptake. 30, 31 Scher et al reported a sensitivity of 87% and a specificity of 62% for the detection of primary prostate cancer with histopathologic examination of the resection specimen or biopsy as reference standard. 32 However, an Italian group of investigators reported a sensitivity of 66% and specificity of 81% for localization of primary prostate cancer on a sextant histopathologic analysis 33 ( ▶ Fig. 11.2). Martorana et al reported a sensitivity of 83% in detection of primary tumor nodules of 5 mm and larger, although the sensitivity for assessment of extraprostatic extension was inferior to MRI (22% for 11C-choline PET vs. 63% for MRI, p < 0.001). 34 Eschmann et al compared 11C-choline PET/CT with whole-body MRI for staging of prostate cancer with histologic analysis and follow-up as validation criteria. 35 The sensitivity and specificity were 97% and 77%, respectively, for 11C-choline PET and 79% and 94% for whole-body MRI. These results suggested that PET and MRI might provide complementary diagnostic information in the initial staging of prostate cancer. Overall, while 11C-choline PET may be helpful in detecting primary prostate cancer, the diagnostic performance may depend on several important factors such as tumor grade, size, and location. 36 Fig. 11.2 Utility of 11C-choline positron emission tomography–computed tomography–guided (PET/CT-guided) biopsy that confirmed tumor in the anterior transition zone of the prostate in a patient with a prior negative standard systematic 12-core biopsy. Axial CT (top left); 11C-choline PET (top right); and fused 11C-choline PET/CT (bottom left). (Reproduced with permission from Farsed et al 2005, 33 Fig. 4.) The potential use of other PET radiotracers in the setting of primary tumor detection and initial staging is unsettled in view of the paucity of published reports. There is one case report of the potential use of 68Ga-labeled ligand of prostate-specific membrane antigen (PSMA) in the initial diagnostic setting. 37 However, another recent case report, also using this tracer, has indicated limitations, in particular, false-negative results in the poorly differentiated prostate cancer with neuroendocrine differentiation. 38 We recently reported a clinical case example of a patient with elevated serum PSA level of 10.5 ng/mL and a prior negative standard TRUS-guided biopsy who underwent a clinical 3-T multiparametric MRI and a research protocol PET/CT with the thymidine analog cellular proliferation radiotracer 18F-FMAU (2′-deoxy-2′-[F]fluoro-5-methyl-1-β-D-arabinofuranosyluracil). The PET/CT and multiparametric MRI were fused with the TRUS for real-time combined image-based targeting of the biopsy needle to an area with abnormal tracer localization, which on histopathology revealed early malignancy. 39 It is anticipated that either through integrated PET/MRI or software-fused PET and MR images of the prostate, there will be clinical utility not only in detection and localization of prostate cancer (for targeted biopsy) but also in image characterization (indolent vs. aggressive) of tumor sites. 40, 41, 42, 43, 44, 45 Fusion of imaging data obtained from 11C-acetate PET/CT and DCE-MRI at 1.5T has been shown to provide a competitive advantage over each separate imaging modality alone. 46 Park et al introduced a combined PET/MRI–derived parameter, the tumor-to-background ratio of each voxel′s 11C-choline SUV divided by its ADC, that was noted to be significantly different between prostate cancer with a Gleason score ≥ 3+4 and prostate cancer with a Gleason score ≤ 3+3. The authors suggested that this PET/MRI–derived parameter might be able to characterize prostate lesions. 47 Image-guided biopsy may be performed by either a direct MRI-guided approach or through dynamic fusion of MRI and TRUS images. Hartenbach et al reported that combined 18F-fluoroethylcholine PET/MRI showed statistically significant higher accuracy in detection of the dominant malignant lesion in the prostate gland when compared to either PET or MRI alone. 48 Kim et al reported on the diagnostic performance of simultaneous PET/MRI with 18F-fluorocholine in 30 patients with localized prostate cancer prior to radical prostatectomy. 49 MRI, 18C-fluorocholine PET, and combined PET/MRI, all evaluated based on the single simultaneous PET/MRI acquisition, identified the sites of the prostate tumor in 83.3%, 80%, and 93.3% of cases, respectively. The authors concluded that combined 18F-fluorocholine PET/MRI demonstrated an improved diagnostic performance over either modality alone. Although this study was performed using a simultaneous PET/MRI imaging system, the unique utility of simultaneity was not described. However, in this context, Rosenkrantz and colleagues showed that dynamic analysis of FDG PET data obtained during simultaneous PET/MRI data acquisition might be useful in localization of small prostate tumors. 50 Wetter et al showed that SUVs obtained from 18F-fluorocholine PET/MRI were significantly lower than those obtained with PET/CT, probably related to differing attenuation correction schemes. 51, 52, 53 Invasive treatment for localized disease (radical prostatectomy or radiation therapy) is done with the intent to cure. However, up to 35% of patients (or higher in select high-risk groups) may experience biochemical recurrence (PSA relapse) within a decade of primary definitive therapy. 54 Localization of disease in this group of patients is pivotal as it directs appropriate management, which may include salvage therapy (surgery or radiation) for local recurrence and systemic therapy for metastatic disease, or both. Biochemical failure is defined as an increase in serum PSA level with negative standard imaging studies after definitive therapy for primary prostate cancer. The American Urological Association (AUA) defines biochemical recurrence in post-prostatectomy patients as an initial serum PSA level of 0.2 ng/mL or higher, with a second confirmatory level higher than 0.2 ng/mL. 55 The American Society for Therapeutic Radiology and Oncology consensus definition for biochemical failure after primary external-beam radiation therapy is an increase of 2 ng/mL or more above the nadir PSA level, regardless of hormonal therapy. 56 In general, FDG PET appears to have a limited role in this clinical setting, although higher PSA levels may be associated with higher probability of detection of metabolically active disease. In one study, FDG PET demonstrated a sensitivity and specificity of 75% and 100%, respectively, for the detection of pelvic lymph node metastases, with validation based on histopathologic examination of the surgically harvested nodes. 57 We have reported our findings of a prospective investigation on the potential utility of FDG PET/CT and 18F-NaF PET/CT in detection of occult metastases in 37 men with PSA relapse (range, 0.5–40.2 ng/mL) and strictly negative standard imaging studies. 58 18F-FDG PET/CT only was positive in 1 patient, 18F-NaF PET/CT only was positive in 8 patients, and both were positive in another 2 patients. Overall, we found a detection rate of 8.1% for FDG PET/CT in the setting of biochemical recurrence. In another investigation, although not specific to prostate cancer, Eiber et al compared whole-body integrated PET/MRI with PET/CT for evaluation of bone lesions. 59 Given that most metastases from prostate cancer occur in the skeleton, and that skeletal metastases are a major source of morbidity in this disease, the detection, localization, and evaluation of extent of osseous lesions by imaging are of prime importance. These investigators found that fully integrated whole-body FDG PET/MRI was superior to PET/CT for anatomical demarcation and localization of bone lesions. It remains to be seen if PET/MRI has a competitive advantage over PET/CT in detection of disease sites in biochemical recurrence of prostate cancer. Most studies with 11C- and 18F-choline in prostate cancer have been in the biochemical recurrence phase of the disease 60 ( ▶ Fig. 11.3). Umbehr et al provided a systematic review and meta-analysis of 11C- and 18F-choline in restaging patients with biochemical recurrence. They reported, on a per patient basis (12 studies, 1,055 patients), a pooled sensitivity and specificity of 85% (95% CI, 79–89%) and 88% (95% CI, 73–95%), respectively. 61 A similar report by von Eyben et al examined 47 articles and data from 3,167 patients with regards to the diagnostic utility of choline PET/CT in staging and restaging of prostate cancer. 62 They found that there was a statistically significant greater number of positive results in the prostate bed of biochemically relapsed patients who had previously undergone external-beam radiation therapy than in patients who had radical prostatectomy as the initial treatment. Moreover, choline PET/CT led to a change in treatment in 381 of 938 (41%) patients, leading to a complete response of PSA to treatment in 101 of 404 (25%) patients. Another systematic review and meta-analysis by Evangelista and colleagues (19 studies, including 12 studies for all sites of disease, 3 for lymph node metastases, and 4 for local recurrence; 1,555 patients) on the use of choline PET and PET/CT in biochemical relapse of prostate cancer reported a pooled sensitivity of 85.6% (95% CI: 82.9–88.1%) and pooled specificity of 92.6% (95% CI: 90.1–94.6%) for all sites of disease (prostatic fossa, lymph nodes, and bone), a pooled sensitivity of 75.4% (95% CI: 66.9–82.6%) and pooled specificity of 82% (95% CI: 68.6–91.4%) for prostatic fossa recurrence, and a pooled sensitivity of 100% (95% CI: 90.5–100%) and pooled specificity of 81.8% (95% CI: 48.2–97.7%) for lymph node metastases. 63 The reported 100% pooled sensitivity for detection of lymph node metastases may have been overestimated given the small number of publications that were included in the meta-analysis. Fig. 11.3 A 60-year-old man with a history of prostate cancer who had undergone tumor resection and presented with a rising serum prostate-specific antigen level. Right column, from top to bottom, shows 18F-fluorocholine positron emission tomography (PET), pelvic computed tomography (CT), and fused PET/CT images demonstrating abnormal accumulation of radiotracer in a normal-sized right internal iliac lymph node (arrows). Maximum-intensity–projection image on the left shows normal biodistribution of 18F-fluorocholine and no other suspicious lesions. (Image courtesy of Dr. Mohsen Beheshti, St. Vincent’s Hospital, Linz, Austria. Reproduced with permission of Jadvar et al 2011. 17)
11.2 Primary Diagnosis and Staging
11.3 Biochemical Recurrence and Restaging
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree