© Springer-Verlag Berlin Heidelberg 2014
Kyung-Hoi Koo, Michael A. Mont and Lynne C. Jones (eds.)Osteonecrosis10.1007/978-3-642-35767-1_3535. Is There a Role for BMPs in the Treatment of Osteonecrosis?
(1)
Department of Orthopaedic Surgery, Stanford University Medical Center Outpatient Center, 450 Broadway St. M/C 6342, Redwood City, CA, 94063, USA
(2)
Department of Orthopaedic Surgery and Bioengineering, Stanford University Medical Center Outpatient Center, 450 Broadway St. M/C 6342, Redwood City, CA, 94063, USA
35.1 Introduction
The cause for osteonecrosis (ON) is still largely unknown and might in fact be multifactorial [1–3]. Multiple theories exist and almost all lead to impaired osseous blood flow with subsequent bone and marrow cell death. In cases of steroid- and alcohol-induced ON, current research points towards a predominance of adipogenic over osteogenic differentiation of mesenchymal stem cells, leading to osteocyte death and impaired remodeling with fatty atrophy of the subchondral bone [4, 5]. The foundation for the vascular insult theory is thought to be either from a traumatic disruption of the vasculature, from stenosis (i.e., compression), or from occlusion (i.e., thromboembolic event) [6]. After the initial ischemic event, necrosis of the cancellous bone and adjacent bone marrow ensues. This leads to an activation of signaling pathways that cumulate in an inflammatory response. Macrophages and osteoclasts home to the site of acute necrosis and initiate a resorption phase. In cases where only a small area of bone is involved, creeping substitution will replace the necrotic bone and the process will likely resolve without any clinically significant sequelae. If, however, the area of necrosis exceeds the size that can be regenerated through creeping substitution, then osteoclastic resorption can lead to structural compromise with the detrimental result of collapse. At this final stage of osteonecrosis, regenerative treatment options are futile and replacement of the involved joint is most commonly the last option. It is estimated that about 5–12 % of total hip arthroplasties are performed for sequelae of osteonecrosis of the femoral head [7, 8]. Current research focuses on the development of regenerative strategies that could lead to an osteogenic response during the pre-collapse state. Growth factors are the main target of this research. If we understand how to control the choice between proliferation and differentiation, then theoretically we will have the ability to expand what might be a limited population of progenitor cells within the site of osteonecrosis, induce their timely differentiation, and thus restore the function of the involved bone segment.
Recent publications suggest that BMPs are among the most attractive targets for such therapeutic interventions [9]. In this chapter, we will introduce the concept of BMP-induced bone regeneration during the repair of osteonecrotic lesions and will discuss current preliminary basic science and clinical trials.
35.2 Osteonecrosis and Bone Morphogenetic Proteins
Bone morphogenetic proteins (BMPs) were discovered by Marshall Urist in 1965 [10]. Since then, seven BMPs have been identified; six of these belong to the transforming growth factor beta superfamily [11, 12]. BMPs have become the focus of recent investigations with the goal to stimulate bone formation by adding exogenous growth factors. It is now clear that BMPs achieve their dramatic effect by inducing cells to adopt a chondrogenic fate and depositing an extracellular matrix rich in proteoglycans [13]. The resulting cartilaginous callus is then slowly replaced by bone through the process of endochondral ossification, which appears to be independent from the BMP response [12]. Besides this best known and understood role, BMPs play a role in a variety of cell fate decision involving chondrocytes, osteoblasts, and osteoclasts. The latter have been the source of recent debate, as new data showed a BMP-dependent activation of osteoblast-dependent osteoclastogenesis via the RANKL-OPG pathway. This increased osteoclastogenesis results in bone resorption rather than bone deposition, and therefore, a revision of the traditional understanding of the BMP pathway in clinical therapeutics may be warranted [14, 15].
Currently there are two BMP formulations (BMP-2 (Infuse, Medtronic) and BMP-7 (OP-1, Stryker)) approved by the FDA for treatment of acute open fractures of the tibial shaft, for spinal fusion procedures (BMP-2), and for treatment of recalcitrant long bone nonunions (BMP-7). The use of BMPs in orthopedic surgery otherwise is off-label. We have searched the current literature for evidence suggesting that BMPs have been used for the treatment of pre-collapse osteonecrosis.
As described above, osteonecrosis is thought to be the result of a microvascular insult, which then leads to bone and marrow necrosis. This sequence of pathologic events offers three distinct targets as therapeutic approaches: lack of (1) angiogenesis, (2) viable progenitor cells within the defect, and (3) osteogenic stimulus to the few remaining progenitor cells in the periphery of the lesion.
Cui et al. proposed an experiment in which all three deficits were targeted with a simple experimental strategy [16]. They suggest the use of genetically engineered bone marrow stromal cells, carrying VEGF and BMP-6 genes, to enhance angiogenesis and osteogenesis in necrotic bone using a chicken ON model . By delivering genetically engineered progenitor cells, they not only supply the needed growth factors but also the cellular machinery required to establish the bony regenerate. Unfortunately, the results of these proposed experiments are not yet published and therefore can only provide basis for speculations. As with all gene therapy approaches, this idea will need rigorous preclinical testing followed by extensive clinical trials prior to an approval for the common treatment of osteonecrosis.
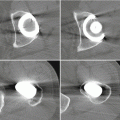
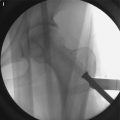
There are, however, attempts of mimicking and simplifying the abovementioned approach by administering the purified protein into the area of osteonecrosis. Recent data suggest that BMP not only induces osteogenesis but also acts as a very potent stimulator of angiogenesis [17]. This effect is achieved through osteoblast-derived vascular endothelial growth factor A expression. Thus, addition of BMP to the site of osteonecrosis would potentially induce angiogenesis through the effect of VEGF expression, followed by increased osteogenesis , which is a direct BMP-dependent mechanism. This strategy addresses two of the three main therapeutic hurdles that we are facing in the treatment of ON. Wang et al. published their results using this approach in a murine model of ON [18]. In detail, they set out to test whether rhBMP2 was able to penetrate into the area of necrosis and, if so, could promote angiogenesis and creeping substitution of the nonviable bone. They first tested three different rhBMP-2 carriers, all containing hydroxyapatite and poly (D,L-lactide-co-glycolide)(PLGA65/35), in which the BMP protein is either contained within microspheres or tethered to the outside of spheres at different concentration. By using a murine model, in which a fragment of necrotic bone was interposed into a tibial defect, they showed that the higher concentration of rhBMP-2 (2,000 ng) tethered to the outside of the microsphere lead to a daily release of approximately 80 ng/ml per day. This release concentration resulted in a strong angiogenic response within the necrotic bone and a subsequent solid callus formation around the fragment. Despite this significant healing response in this model, there are clear differences between this model and the clinical presentation of ON. ON of the subchondral bone presents a slightly different environment than the tibial defect with intercalary necrotic segment model that is presented by the authors. There is abundant evidence that suggests that BMPs act on osteochondral progenitor cells that reside within the cambial layer of the periosteum [13, 19], but until now, there is no published evidence that BMPs activate osteoprogenitor cells within the bone marrow. Considering this caveat, other experimental designs that mimic the condition of osteonecrosis more closely will be needed.
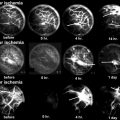
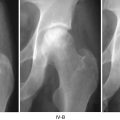
Several animal models for osteonecrosis exist, but only a few recapitulate the various aspects of the early histopathological features combined with the late macroscopic collapse. Experimental models in goat [20], rabbit [21], and emu [22] have been published, and all have in common that osteonecrosis was created by ligation of the vascular supply to the femoral head in addition to deep-freezing of the cancellous bone of the femoral head with liquid nitrogen. In these models, ON occurs reliably and leads to subsequent collapse of the subchondral bone. This feature allows the investigators to not only investigate the early effect of BMP treatment but also to ensure that the BMP-induced bone regenerate provides a mechanically stable scaffold for maintenance of joint congruity. Tang et al. [23] employed the abovementioned goat model to evaluate if hBMP-2-gene-modified bone marrow stromal cells can be utilized for the treatment of ON of the femoral head. Three weeks after establishment of ON in the femoral head, core decompression was performed, and the defect was filled with either β-tricalcium phosphate loaded with BMP-2-gene-modified bone marrow mesenchymal stem cells or β-TCP with β-gal-gene-modified bone marrow mesenchymal stem cells. Sixteen weeks after surgery, femoral heads in both groups maintained their original shape without any signs of collapse. Histology revealed lamellar bone formation in the BMP group, compared to some fibrous tissue in the β-gal group. Biomechanical analysis revealed a significant difference between the two groups with a higher maximum compressive strength and Young’s modulus in the BMP-2 group. This study provides sound evidence that BMP-2 gene therapy provides a feasible, and most importantly, successful approach in the prevention of subchondral collapse due to ON.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree