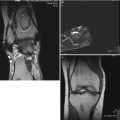
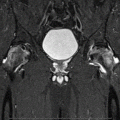
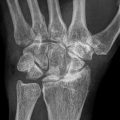
Fig. 61.1
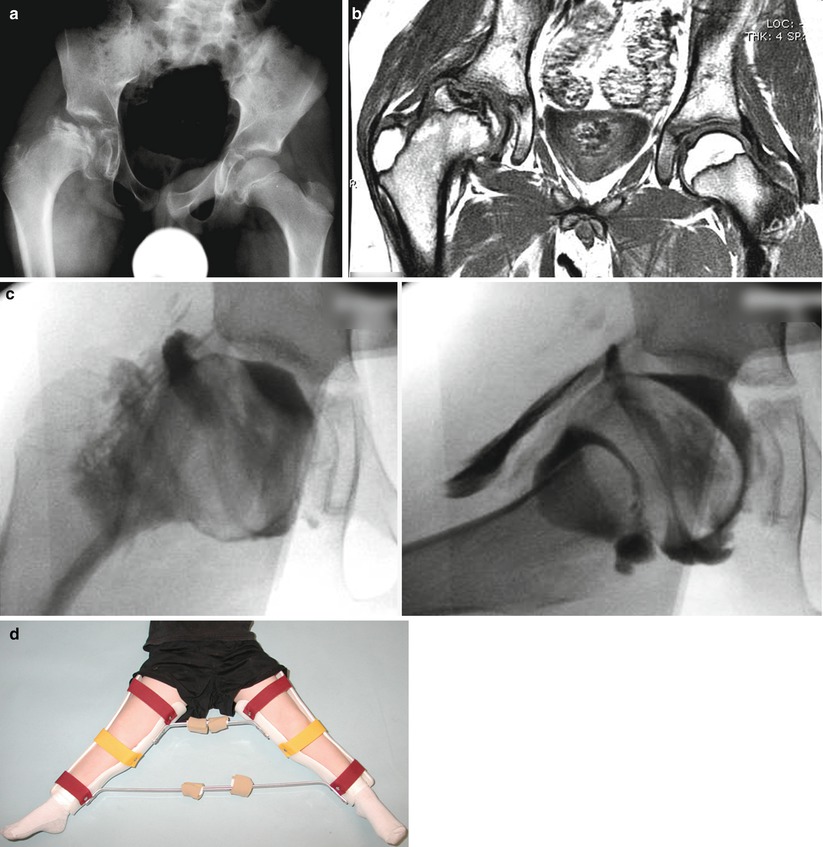
Eleven-year and 7-month-old male with right hip pain for 12 months. (a) AP pelvis and frog-leg lateral radiographs showing right hip LCPD in Waldenstrom stage 2A (early fragmentation stage) with some flattening of the superior region of the femoral head. The lateral pillar classification cannot be applied reliably at this stage. (b) Gadolinium-enhanced MRI with subtraction technique (perfusion MRI) showing an absence of perfusion in the central and lateral regions of the femoral head (>50 % of epiphysis was hypoperfused). This patient was treated with early femoral varus osteotomy (10–15° varusation) and prolonged toe-touch weight bearing postoperatively. (c) AP and frog-leg lateral radiographs obtained 3 years postoperatively. The patient was performing sports activities and symptom free
The mechanism by which the varus osteotomy alters the natural history of LCPD is not clear. According to force calculations, reducing the neck-shaft angle from 130° to 110° results in only a 14 % reduction in load across the femoral head [44]. A mathematical model indicated that the compressive forces on the epiphysis is three to four times body weight during normal gait, even after a varus osteotomy [45]. It remains unclear if the osteotomy stimulates or alters the healing process, as studies have conflicting results [12, 38, 46–48]. Potential beneficial effect of varus osteotomy may be a combination of biological effect, alternation of the hip biomechanics, and forced decrease in the activity level as a result of the surgery.
While controversial, femoral osteotomy performed in the initial stage or early fragmentation stage seems to better maintain the sphericity of the femoral head [12, 26, 38, 49]. Axer et al. [49] noted that only 9 % of patients had a poor result if operated on during the initial necrotic stage compared to 14 % during the intermediate fragmentation stage and 56 % treated in late regenerative stage. In a more recent study, 97 patients in different stages of disease were analyzed and the only factor under physician control that altered the femoral head sphericity was timing of surgery [11]. The same group confirmed this is a larger retrospective study of their patient population of 640 patients [38]. A concern previously raised is the possibility of proximal femoral physeal closure if the osteotomy is performed too early in the course of disease [50] and the possibility of operating on a patient with good prognosis who does not need treatment as it is difficult to stratify who needs surgery vs. who does not based on X-rays obtained at the initial stage.
A potential benefit of performing the varus osteotomy in the initial stage of LCPD is bypassing the fragmentation stage of disease and shortening the overall duration of disease [38, 49, 51, 52]. This decreases the time the femoral head is fragile and at risk for deformation. Of 314 patients treated with a femoral varus osteotomy, 34 % bypassed the stage of fragmentation compared to only 1 of 40 who were treated nonoperatively [38]. It is important to note that the postoperative regimen included a prolonged duration of non-weight bearing (personal communication from the senior author). The results from this study do contradict older reports that femoral osteotomy does not alter the rate of disease progression [48] but the timing of intervention and postoperative regimen may have been very different between these studies. Incomplete or “biologic” osteotomies have also been attempted without effect on the duration of disease [46, 47].
Some concerns raised about femoral varus osteotomy are the reduction of the abductor lever arm which can potentially worsen a limp, shortening of the affected limb, and worsening of the overriding greater trochanter and lateral impingement. These potential problems are dependent on the amount of varusation and the functional state of the proximal femoral physis. Unfortunately the minimum amount of varus necessary to be effective in altering the natural history of LCPD is not known. It is important to contain the lateral pillar of the head under the acetabulum, and a patient in a later stage of disease with greater femoral head deformity may need more varus than a patient in the initial stage of disease with minimal deformity. The femoral neck-shaft angle does remodel after varus osteotomy [53–55] in about 65 % of the patients with most improvement occurring in the first 2–3 years [56]. However, this is dependent on the health of the proximal femoral physis, which is difficult to predict using plain radiography and some patients simply do not remodel at all [55, 57]. A recent study of patients who had varus osteotomy in the early stage of LCPD found that larger degrees of varus angulation was not associated with better Stulberg outcomes leading the authors to recommend a mild to moderate 10–15° varus osteotomy when performed in the early stage of LCPD [55].
Some surgeons advocate performing an epiphysiodesis of the greater trochanter at the time of femoral varus osteotomy or at the time of hardware removal if it is planned within the first postoperative year [43]. The goal is to avoid a relative trochanteric overgrowth after a varus osteotomy, especially if a growth arrest or disturbance of the proximal femoral physis is present. The beneficial role of the trochanteric epiphysiodesis and the optimal timing of the procedure is not clearly defined at this time.
Complications of femoral varus osteotomy include excessive proximal femoral varus, failure of varus angulation to remodel, limb shortening, limp, overriding greater trochanter with lateral impingement, and widened appearance of the hips and fracture after hardware removal [54]. If the hardware is to be removed, it should be done at 6 months or later [58].
61.4.2 Pelvic Osteotomy
Salter’s innominate osteotomy was initially developed for the treatment of congenital hip dislocations [59]. Salter applied this treatment to LCPD to surgically contain the femoral head when extrusion was present [60]. This was analogous to treating a dysplastic hip with residual subluxation. He used this operation in children over 6 years old with moderate to severe involvement and loss of containment. Contraindications included patients with femoral head deformity on arthrogram and significant limitation of motion [61]. Threaded pins were used to avoid postoperative casting and generally removed at 6 weeks postoperatively [18]. Patients were allowed full weight bearing and activities at 6 weeks once the osteotomy healed. Salter and other authors have reported improved outcome with the osteotomy over the natural history of LCPD [18, 61].
Proposed advantages of Salter’s innominate osteotomy are that it does not alter the length of the hip abductor muscles nor shorten the limb as with a varus femoral osteotomy. In general, the functional advantage of the Salter’s osteotomy over the femoral osteotomy is difficult to demonstrate, but in severe cases where physeal closure is expected, the Salter’s osteotomy leads to a smaller leg length discrepancy [62]. Theoretically, the Salter’s osteotomy can lengthen the limb, leading some surgeons to modify the osteotomy by recessing the distal fragment into the intact ilium [63]. Complications of the Salter osteotomy include loss of acetabular position, failure of fixation, decreased range of motion, and impingement. Recent recognition of a high prevalence of acetabular retroversion in patients with LCPD has raised a concern about performing an osteotomy that may further contribute to the acetabular mal-orientation and femoroacetabular impingement [64–66]. The validity of this concern remains unclear. Iatrogenic hinged abduction can occur if the Salter does not completely contain the femoral head [67].
61.4.3 Combined Osteotomies and Triple Pelvic Osteotomy
For severe disease, some have recommended a combined femoral varus and Salter osteotomy to maximize the coverage of the necrotic femoral head while balancing the disadvantages of each operation [68–72]. The amount of varus can be minimized to avoid limb shortening and abductor weakness. The Salter osteotomy correction does not have to be as large decreasing the risk of impingement. The clinical merit of the combined osteotomy approach compared to single femoral or pelvic osteotomy has yet to be determined.
The triple pelvic osteotomy has also been used in the treatment of LCPD [71, 73–76]. The goal is to provide an adequate femoral head coverage, while avoiding a femoral osteotomy. More coverage can be obtained, because the acetabulum is completely freed from the remainder of the pelvis [71]. Wenger et al. [71] reported on 40 hips in 39 children, with 42 % of patients having a Stulberg I or II result and 58 % a III, IV, or V. When broken down further, 65 % of lateral pillar B hips and 12 % of C hips had a Stulberg I or II result. Four patients required reoperation, either femoral head osteochondroplasty or valgus osteotomy, for hinged abduction. The radiographic rate of pincer impingement due to over coverage was high [77].
61.4.4 Shelf Acetabuloplasty
The goal of shelf acetabuloplasty is to provide added acetabular coverage over an uncovered femoral head. Originally this was used as a salvage procedure to cover deformed femoral heads with coxa magna, subluxation, or hinged abduction. The indication for this procedure typically occurred in the later stages of disease, and the procedure was therefore used after attempted nonoperative treatment [78]. However, some advocated the use of shelf acetabuloplasty as a primary containment treatment and an alternative to a femoral or innominate osteotomy [79–81]. The proposed benefit of this procedure is that the shelf protects the labrum and stimulates acetabular growth providing femoral head coverage and preventing the development of the femoral head deformity [79, 81, 82].
Various techniques for shelf acetabuloplasty have been reported including a minimally invasive one [79, 83]. Care should be taken to ensure that the graft sits directly on the capsule for the shelf to form appropriately and function as a support. The ideal location is in line with the subchondral bone of the acetabulum. Postoperatively, a spica cast is used and protected weight bearing is recommended while the incorporation of the graft occurs. Complications of the shelf procedure include resorption of the graft (often if placed too high), inadequate coverage, excessive coverage, and impingement.
No prospective study evaluating the shelf procedure for LCPD has been performed. In one study, there was no evidence to indicate that the procedure prevents the development of early osteoarthritis [84].
61.4.5 Hip Arthrodiastasis
Hip arthrodiastasis can be considered as a form of weight relief while allowing limited hip motion. The indications for hip arthrodiastasis and the best time to institute this form of treatment remain unclear. In general, the procedure is reserved for older children with a poor prognosis (typically over 8 years old) or those with significant collapse of the femoral head and a stiff hip. Most reports are small case series [85–87]. A few series reported promising early results with respect to femoral head height during the stage of fragmentation [86, 87]. However, a follow-up study on a set of those patients at skeletal maturity indicated that seven out of ten patients had a Stulberg IV hip [88].
From the available evidence, arthrodiastasis seems to increase hip range of motion and decrease pain. There is no literature to support that it has a beneficial effect on the shape of the femoral head at maturity at this time. Some surgeons believe that it speeds up the rate of healing, but prospective studies have not been performed to support this anecdotal observation. Some limitations of this procedure include pin site infection, loosening, pain that restricts the duration of the treatment, and the need for extensive physiotherapy to gain and maintain hip motion.
61.5 Current Treatment Algorithm for Legg-Calvé-Perthes Disease
61.5.1 Current Best Evidence
Our current treatment algorithm is based on two prospective studies (level 2 evidence) [26, 30] and two meta-analyses with the age at onset of symptoms as a primary factor in decision-making in conjunction with the Waldenström stage, the amount of head necrosis based on perfusion MRI, and the lateral pillar classification if patient presents at the mid-fragmentation stage. Herring et al. [26] reported on 451 hips in 438 patients older than 6 years as a part of a multicenter prospective study. The study was conducted as a “best effort” design such that surgeons chose one of five specific treatments (no treatment, range of motion, brace, innominate osteotomy, or femoral osteotomy) for all his or her patients. Comparisons were made between nonoperative and operative treatments as there were no statistical differences when comparisons were made within each group. For the patients with onset of the disease between age 6 and 8 years, the operative group did not show a statistically significant improvement in the outcome. However, the percent of patients with Stulberg I or II outcomes were higher in the operative groups compared to the range of motion group (Table 61.1). Operative treatment significantly correlated with improved Stulberg outcome in patients over 8 years old with lateral pillar B or B/C hips (Table 61.2). Seventy-three percent of patients over 8 years old in the lateral B group treated surgically had a Stulberg I or II outcome at skeletal maturity compared to only 44 % treated nonoperatively. No difference was found between operative and nonoperative treatment for lateral pillar A or lateral pillar C hips. It is important to note that 115 of the 120 surgically treated hips were operated on during the initial stage or early fragmentation stage, before the lateral pillar classification can be reliably applied (i.e., surgeons did not wait to classify before instituting treatment) [89] which makes the interpretation of the results based on the lateral pillar groups difficult.
Table 61.1
Outcomes for hips in patients aged 6–8 years at onset in the lateral pillar B and B/C border groups
Stulberg classification | |||||
|---|---|---|---|---|---|
Group | Total | I or II | III | IV or IV | p value |
Lateral pillar group B, age 6–8 years | |||||
Nonoperative | 80 | 61 (76 %) | 17 (21 %) | 2 (3 %) | 0.99 |
Operative | 43 | 33 (77 %) | 9 (21 %) | 1 (2 %) | |
Lateral pillar group B/C, age 6–8 years | |||||
Nonoperative | 21 | 6 (29 %) | 10 (48 %) | 5 (24 %) | 0.1 |
Operative | 14 | 9 (64 %) | 4 (29 %) | 1 (7 %) | |
Table 61.2
Outcomes for hips in patients older than 8 years at onset in the lateral pillar B and B/C border groups
Stulberg classification | |||||
|---|---|---|---|---|---|
Group | Total | I or II | III | IV or IV | p value |
Lateral pillar group B, age >8 years | |||||
Nonoperative | 62 | 27 (44 %) | 26 (42 %) | 9 (15 %) | 0.02 |
Operative | 33 | 24 (73 %) | 7 (21 %) | 2 (6 %) | |
Lateral pillar group B/C, age >8 years | |||||
Nonoperative | 15 | 2 (13 %) | 4 (27 %) | 9 (60 %) | 0.05 |
Operative | 11 | 0 | 8 (73 %) | 3 (27 %) | |
Wiig et al. [30] performed a nationwide prospective study of 368 patients (all unilateral) treated in Norway. Hips were classified based on a two-group version of the Catterall classification (<50 and >50 % involvement) to improve the interobserver agreement. The lateral pillar classification was also studied. Patients with <50 % involvement were treated with physiotherapy alone. Patients with >50 % involvement were treated with physiotherapy, a Scottish Rite orthosis, or a proximal femoral osteotomy. Surgeons had to choose one of these three treatments for all patients under their care. Children under 6 years of age were included. While the paper did not specifically state the timing of surgery, the patients had femoral varus osteotomy at the mid-fragmentation stage when the lateral pillar classification can be applied (personal communication from Dr. Wiig). Outcomes were based on the Stulberg classification during the healed stage (5-year follow-up) and not at skeletal maturity. Only 15 % had closure of one or both triradiate cartilages and only 14 % had closure of one or both proximal femoral physes. An impressive 97 % of patients returned for follow-up at 5 years. The strongest predictor of outcome was femoral head involvement followed by age. Surgery resulted in statistically significant improvement in the modified Stulberg outcome in patients over 6 years of age with >50 % head involvement (43 % Stulberg I and II) compared to therapy (33 %) and brace treatment (20 %) (Table 61.3). The effect size of the surgery, however, was relatively modest compared to physiotherapy. No stratified analysis of patients between the ages of 6 and 8 was performed. The authors recommended a proximal femoral varus osteotomy for patients over 6 years of age at the time of diagnosis with >50 % head involvement.
Table 61.3
The Stulberg outcomes for patients over the age of 6 years old with >50 % of femoral head necrosis
Stulberg classification | ||||
|---|---|---|---|---|
Group | Total | I or II | III | IV or IV |
Physiotherapy | 51 | 17 (33 %) | 14 (27 %) | 20 (40 %) |
Orthosis | 25 | 5 (20 %) | 9 (36 %) | 11 (44 %) |
Varus osteotomy | 70 | 30 (43 %) | 32 (46 %) | 8 (11 %)a |
Two recent meta-analyses support the results of these two prospective studies [90, 91]. Saran et al. [91] reviewed 14 papers meeting their criteria, which included a total of 1,831 hips. Only proximal femoral and Salter osteotomies were reviewed. Surgery improved femoral head sphericity compared to nonoperative treatment by an odds ratio (OR) of 1.29. For children under 6 years old, surgery was not beneficial (OR 1.02). For children over 6, surgery improved femoral head sphericity by an odds ratio of 2.05. Based on the statistics, surgery seemed to be more beneficial in patients over 8. Despite showing a trend toward improving femoral sphericity, the results did not reach statistical significance when a subgroup analysis was performed on patients aged 6–8.
Nguyen et al. [90] included 23 papers totaling 1,266 hips in a meta-analysis. Patients under 6 years of age were equally likely to have a successful radiographic outcome (Stulberg I or II) with operative or nonoperative treatment. However, the patients under age 6 treated with a pelvic procedure had a five times greater chance of a successful outcome than patients treated with a femoral osteotomy. For the patients over 6 years old, operative treatment was almost twice as likely to result in a successful radiographic outcome. There was no difference between a pelvic or femoral procedure in patients over 6 years of age. No difference in outcomes was found between males and females, which contradicts other studies indicating females have a worse prognosis [26].
Surprisingly, these two meta-analyses only shared two articles between them. Some differences in the methodologies of the two studies were as follows. First, slightly different search techniques were used. Second, Saran et al. [91] looked only at femoral and Salter osteotomies, while Nguyen et al. [90] included femoral osteotomy, innominate osteotomy, shelf acetabuloplasty, Chiari osteotomy, and combined procedures (femoral plus pelvic). Interestingly, the prospective study by Wiig et al. [30] was included in both, while the one by Herring et al. [26] was only included in one meta-analysis.
Given the above evidence, we recommend dividing patients based on age of symptom onset into the following groups – less than 6, 6–8, 8–11, and over 11 years of age. For treatment purposes, we consider patients over the age of 11 to have the worst prognosis. Femoral head ischemia in children this age may be more similar to avascular necrosis of the femoral head in adults which has a poor prognosis if a large area of the femoral head is involved [92–94]. In addition to age, we consider the Waldenström stage and lateral pillar classification, if it can be determined. For patients in the initial stage of disease or early fragmentation, when a lateral pillar classification cannot be reliably determined, we use the extent of femoral head necrosis (i.e., hypoperfusion) on a gadolinium-enhanced (perfusion) MRI [95, 96]. In a preliminary study, perfusion MRI has been shown to provide prognostic information about the disease severity in the initial stages of disease (see Chap. 60 on diagnosis, imaging, and classifications). We incorporate age, stage, extent of involvement, range of motion, and social factors to make a shared decision with parents to determine the best treatment course for each patient.
61.5.2 Treatment for Children Under Age 6
It has long been recognized that age correlates with the long-term outcome of Perthes disease [2, 97–100]. The assumption is that a younger patient has more potential to remodel the deformed shape of the femoral head compared to an older patient. Surgery for children under 6 years of age is unlikely to be beneficial as multiple studies have shown no difference between operative and nonoperative treatments [30, 33, 90, 91]. Unfortunately, not all patients in this group are guaranteed a good outcome.
Rosenfeld et al. [34] reported on 188 hips in 172 patients under the age of 6 at onset. The only operative intervention was hip adductor release and/or iliopsoas release in patients treated with a Petrie cast. Eighty-one percent of patients had a Stulberg I or II result at final follow-up and 19 % a Stulberg III or IV. Canavese et al. [33] reported similar results on 166 hips in patients under 6 years of age. Operative treatment was performed on 57 patients, but no difference was noted between those treated operatively and nonoperatively. Overall, 67 % of patients had a Stulberg I or II hip at skeletal maturity and 33 % had a Stulberg III, IV, or V.
We follow these patients with a clinical examination and AP and frog pelvis radiographs every 4 months in the active phase of LCPD. Treatment is focused on pain relief, activity modification, and maintaining good hip range of motion (Fig. 61.2). Short periods of NSAIDs are used when necessary. For significant loss of hip motion and containment, Petrie casting followed by an A-frame brace and activity restrictions are utilized.
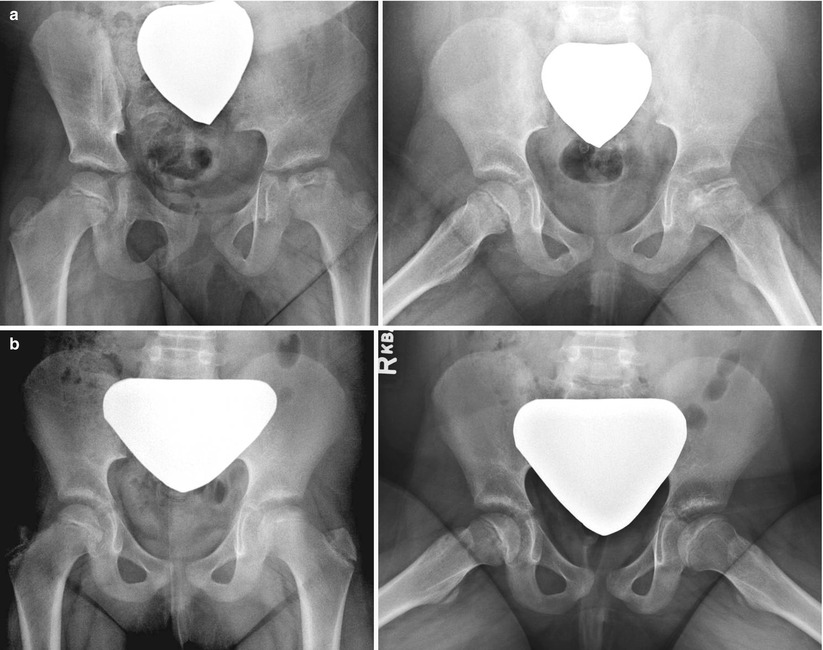

Fig. 61.2
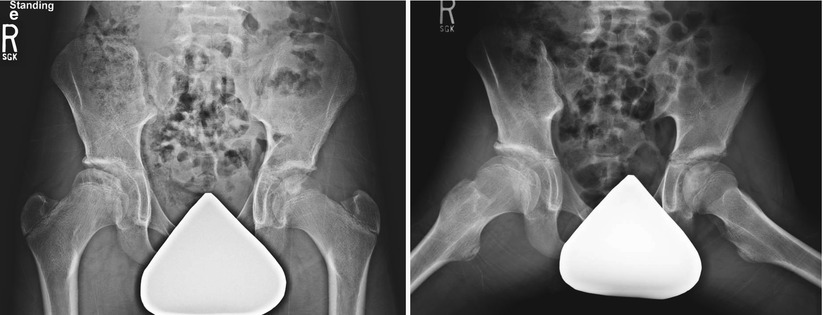
Five-year and 7-month-old female with left hip pain for 1 month. (a) AP pelvis and frog-leg lateral radiographs obtained at mid-fragmentation stage showing lateral pillar B hip. This patient was treated symptomatically and with activity restrictions (no running or jumping). (b) AP pelvis and frog-leg lateral radiographs obtained 3 years and 6 months later. The patient is doing well with full activities and no symptoms
61.5.3 Treatment for Children Aged 6–8
Decision-making for patients presenting between the ages of 6 and 8 years old remains a dilemma since the literature has conflicting reports on the success of surgery over nonoperative measures [26, 30, 90, 91]. One prospective study found an increased chance of a Stulberg I or II outcome with a femoral osteotomy [30], while the other found no difference between operative and nonoperative treatments for this age group [26].
For the patients presenting in the initial stage of the disease, our general approach for this age group is to treat nonoperatively, focusing on managing symptoms, maintaining range of motion, and restricting running and jumping activities. For patients with significant decrease in the hip range of motion (hip abduction less than 15–20°), non-weight bearing on the affected limb or home traction is beneficial. If lateral extrusion is present, we perform a hip arthrogram to determine if the hip can be contained with abduction casts. When abduction with or without an adductor tenotomy provides adequate containment, the patient is placed into a Petrie cast for 6 weeks. When the cast is removed, operative containment procedure (femoral osteotomy) or a nonoperative containment using A-frame orthosis [32] is offered. Other options include prolonged weight relief using crutches or wheelchair.
Perfusion MRI may be useful if surgical treatment is being planned for a patient presenting in the initial or early fragmentation stage of LCPD [96]. Perfusion MRI provides information about the extent of femoral head necrosis in the initial stage when the lateral pillar classification cannot be applied [89]. For patients with >50 % head necrosis (i.e., hypoperfusion), we discuss the option of surgical treatment with families.
For the patients who present in the fragmentation stage, the lateral pillar classification is used to help guide treatment [26]. Lateral pillar A do well without surgery and lateral pillar C hips have poor prognosis regardless of treatment. It is the lateral pillar B and B/C which most likely will benefit from surgical treatment.
While previous strategies to limit weight bearing by bed rest or orthosis have been abandoned [3, 4], there has been a recent revival of the recommendation of non-weight bearing using crutches or wheelchair either as a nonoperative treatment or an integral part of the postoperative protocol. Using an immature pig model, Kim et al. [101] showed that local non-weight bearing after ischemic necrosis of the femoral head resulted in significantly less femoral head deformity than when weight bearing was allowed. This form of treatment is prolonged and requires good compliance. Unfortunately, compliance to non-weight bearing may be a challenge to young, hyperactive patients.
61.5.4 Treatment for Children Aged 8–11
There is a consensus in the literature supporting surgical treatment for patients aged 8–11 years old at onset. Two prospective studies indicate that surgery provides a higher statistical chance of a spherical head at skeletal maturity than nonoperative treatment [26, 30, 90, 91].
The patients who present in the initial stage or early fragmentation stage are a challenge, since the lateral pillar classification cannot be applied reliably to predict the prognosis [89]. Given that operating early provides a better chance at a spherical head [11, 38], we use a perfusion MRI to assess the amount of femoral head necrosis (hypoperfusion). Preliminary studies indicate that perfusion MRI provides an object measure of femoral head involvement and provide information about the prognosis for short-term femoral head deformity [95, 96]. Furthermore, by detecting the patients with good perfusion, this assessment could potentially decrease the number of patients that are “over treated” with surgery. We assume that those with greater than 50 % head involvement have a worse prognosis and may benefit from a surgical treatment. The validity of this assumption needs to be further studied. For those patients with >50 % hypoperfusion at the early stage of LCPD, we recommend a proximal femoral varus osteotomy of 10–15° (Fig. 61.1).
If patients present in the fragmentation stage of LCPD, we stratify treatment based on the lateral pillar classification [26]. Patients with lateral pillar A are treated nonoperatively with symptomatic treatments. Hip arthrogram, possible hip adductor tenotomy, and Petrie casting are considered for patients with lateral pillar C hip with femoral head extrusion (loss of containment) and loss of motion. Following 6 weeks of Petrie casting, prolonged bracing with an A-frame brace [32] (Fig. 61.3) or prolonged non-weight bearing is considered in the lateral pillar C patients to limit additional femoral head deformity [101]. We recommend proximal femoral varus osteotomy for lateral pillar B hips. No cast is applied postoperatively. Some recommend postoperative prolonged non-weight bearing until reossification (Elizabethtown stage IIIb), with unrestricted activity thereafter [12, 101, 102]. Others allow weight bearing after 6 weeks when the osteotomy is typically healed. In the studies by Herring et al. [26] and Wiig et al. [30], a short duration of restricted weight bearing was used with 62 and 43 % patients having Stulberg I or II hips, respectively. If the hip motion is restricted preoperatively, the femoral osteotomy is staged first with a period of rest or Petrie casting to improve hip abduction and to decrease the irritability of the hip joint. Operating on a stiff, irritable hip without staging can lead to persistent stiffness and poor outcome.