Liver, Biliary Tract, and Gallbladder
DISEASES OF THE LIVER
Ultrasonography generally is considered the imaging study of choice for the initial evaluation of pediatric liver disorders. If the sonogram is normal, further radiographic evaluation generally is not required. If sonography cannot yield adequate information or if it suggests a mass, computed tomography (CT) or magnetic resonance imaging (MRI) can be used to improve anatomic delineation. This selection is usually based on personal expertise and equipment availability.
HEPATIC NEOPLASMS
Primary Malignant Neoplasms
The role of imaging in patients with a suspected or known hepatic mass is to characterize the lesion, define its extent and relationship to lobar and segmental anatomy and vascular structures for preoperative planning, and monitor the response to chemotherapy or radiation.
Hepatoblastoma.
Hepatoblastoma is the most common primary liver tumor of childhood, comprising approximately 45% of total liver masses. Median patient age at diagnosis is 1 year. The tumor most often presents as an asymptomatic mass. Other features include abdominal pain, anorexia, weight loss, jaundice, and precocious puberty (related to the secretion of chorionic gonadotropins). Serum alpha-fetoprotein levels are elevated in 80% to 90% of patients. Several conditions are associated with hepatoblastoma, including Beckwith-Wiedemann syndrome, hemihypertrophy, fetal alcohol syndrome, familial polyposis coli, and Gardner syndrome (1,2). The tumor has no association with cirrhosis. Metastases are chiefly to the lungs and porta hepatis and less commonly to brain and skeleton.
Hepatoblastoma is the most common primary liver tumor of childhood.
Hepatoblastoma has no association with cirrhosis.
Pathologically, hepatoblastoma contains small, primitive epithelial cells, resembling fetal liver, and occasionally mesenchymal elements. The tumor is usually unifocal, and the right lobe of the liver is most often affected, but multifocal disease or diffuse infiltration can occur. It has a tendency to invade vascular structures, especially the portal vein.
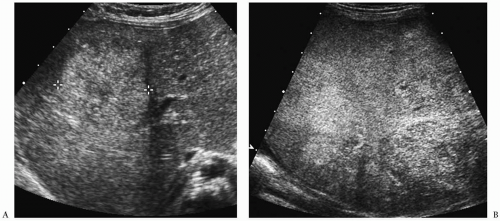
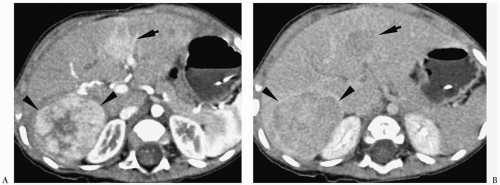
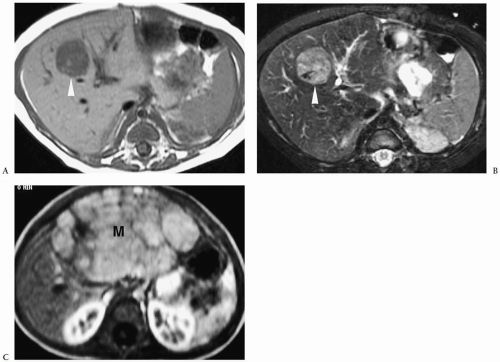
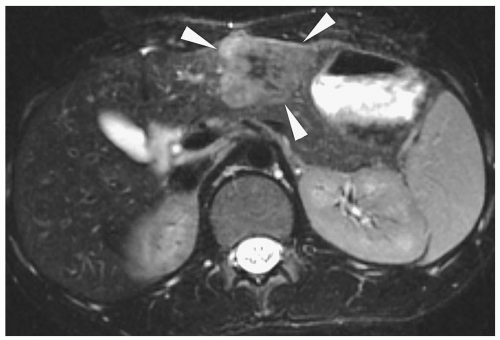
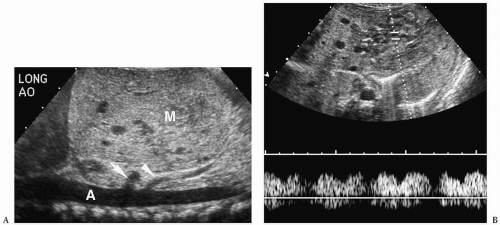
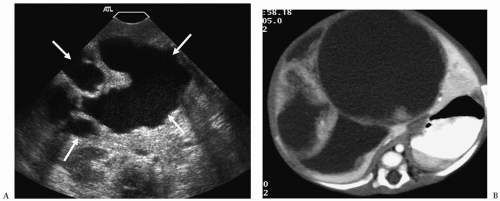
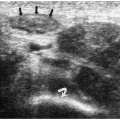
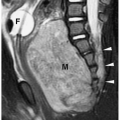
Hepatoblastoma is typically heterogeneous, containing calcifications (50% of tumors) and areas of necrosis (3,4,5,6,7,8,9,10,11). Tumor margins may be well circumscribed or infiltrating. On sonography, hepatoblastoma may be hypo-, iso-, or hyperechoic to normal liver (Fig. 5.1). On noncontrast-enhanced CT, it usually has an attenuation value lower than that of normal hepatic parenchyma, but a small number will be isoattenuating. During the hepatic arterial phase of enhancement, it usually shows transient hyperattenuation relative to normal liver. In the portal venous phase, it commonly becomes hypoattenuating to normal liver (Fig. 5.2). On MR, it generally is hypo- or isointense relative to normal parenchyma on T1-weighted images and hyperintense on T2-weighted and fat-suppressed sequences (Fig. 5.3).
After the administration of gadolinium, it shows an enhancement pattern similar to that seen on CT.
After the administration of gadolinium, it shows an enhancement pattern similar to that seen on CT.
Hepatoblastoma is typically heterogeneous, containing calcification and necrosis.
Secondary findings include spread to portal lymph nodes and intravascular extension. Tumor thrombus appears as an echogenic focus on ultrasonography (US), a low-attenuation filling defect on CT, a high signal attenuation area on spin-echo MRI, and a signal void on gradient echo images.
Hepatocellular Carcinoma.
Hepatocellular carcinoma is the second most common pediatric liver malignancy after hepatoblastoma. In the pediatric population, median patient age is 12 years, with a range of 5 to 15 years (1,2). Pre-existing liver disease, such as hepatitis B infection, type I glycogen storage disease, tyrosinemia, familial cholestatic cirrhosis, hemochromatosis, or alpha-1-antitrypsin deficiency, is present in approximately one half of cases. Serum alpha-fetoprotein levels are elevated in up to 50% of cases (1,2). Abdominal distention and right upper quadrant mass are the most common presenting features.
Hepatocellular carcinoma is the second most common pediatric liver malignancy.
Pre-existing liver disease is common in hepatocellular carcinoma.
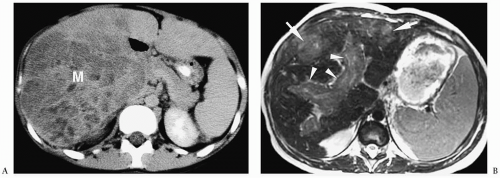
Pathologically, hepatocellular carcinoma contains large, pleomorphic multinucleated cells with variable degrees of differentiation. The tumor is often extensively invasive or multifocal at time of diagnosis. Imaging findings are similar to those of hepatoblastoma (3,4,5,6,7,8,9,10,11) (Fig. 5.4). Differentiation requires tissue sampling.
Fibrolamellar Hepatocellular Carcinoma.
Fibrolamellar hepatocellular carcinoma is a subtype of hepatocellular carcinoma found in older children and adolescents that is less aggressive and has a somewhat better prognosis than the usual hepatocellular carcinoma.
Hepatomegaly and abdominal pain are common presenting features. Serum alphafetoprotein levels are usually normal. Histologically, the tumor contains eosinophilic-laden hepatocytes that are separated into cords by thin, fibrous bands, creating a lamellar pattern, hence, the term fibrolamellar (1,12,13).
Hepatomegaly and abdominal pain are common presenting features. Serum alphafetoprotein levels are usually normal. Histologically, the tumor contains eosinophilic-laden hepatocytes that are separated into cords by thin, fibrous bands, creating a lamellar pattern, hence, the term fibrolamellar (1,12,13).
Fibrolamellar hepatocellular carcinoma is found in older children and adolescents.
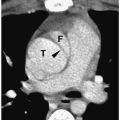
On sonography, CT, and MRI, fibrolamellar hepatocellular carcinoma appears as a well-defined mass that may have a central scar, which can calcify. The tumor is echogenic on sonography, low attenuation on noncontrast CT, hypointense on T1-weighted images, and hyperintense on T2 and fat-suppressed images (Fig. 5.5). Enhancement patterns are similar to those of hepatoblastoma and hepatocellular carcinoma. The central scar has a low attenuation on noncontrast CT and is hypointense on T1- and T2-weighted images. The scar does not enhance.
Fibrolamellar hepatocellular carcinoma appears as a well-defined mass that may have a central scar.
Undifferentiated Embryonal Sarcoma.
Undifferentiated embryonal sarcoma (also known as malignant mesenchymoma and hepatic mesenchymal sarcoma) is a malignancy that primarily affects older children and adolescents. More than 50% are diagnosed in patients between 6 and 10 years of age, and 90% occur by 15 years of age (1). Grossly, the tumor ranges from 7 to 20 cm in diameter and contains cystic spaces and cellular areas. Histologically, it contains primitive undifferentiated spindle cells within a myxoid matrix (1). It
is thought to be the malignant counterpart of mesenchymal hamartoma of the liver (see below). The usual presenting features are abdominal mass and pain.
is thought to be the malignant counterpart of mesenchymal hamartoma of the liver (see below). The usual presenting features are abdominal mass and pain.
Undifferentiated embryonal sarcoma affects older children and adolescents.
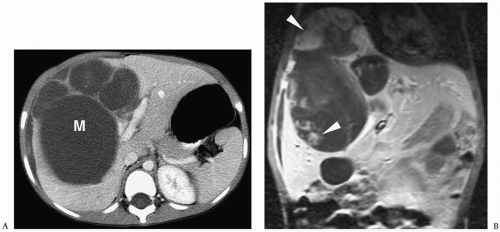
On sonography, CT, and MRI, embryonal sarcoma most commonly appears as a predominantly cystic mass with multiple septations of varying thickness (Fig. 5.6), but on occasion it can present as a predominantly solid mass with multiple small cystic spaces (14). The soft tissue components enhance after the administration of intravenous contrast agents. The imaging features of undifferentiated embryonal sarcoma and benign mesenchymal hamartoma overlap. Metastases are to lung and bone.
Embryonal sarcoma usually appears as a cystic mass with multiple septations.
Secondary Hepatic Neoplasms
Hepatic Metastases.
The malignant tumors of childhood that most frequently metastasize to the liver are Wilms tumor, neuroblastoma, and lymphoma. Neuroblastoma may affect the liver in either stage IV or IV-S disease. Stage IV disease is characterized by the presence of distant metastases to skeleton, liver, or nodes. Stage IV-S neuroblastoma occurs in patients under 1 year of age, who have small ipsilateral tumors (not crossing the midline) and metastases to liver, skin, and bone marrow but not to cortical bone. Clinically, patients present with hepatomegaly, jaundice, abdominal pain or mass, or abnormal hepatic function tests.
Tumors that most often metastasize to the liver are Wilms tumor, neuroblastoma, and lymphoma.
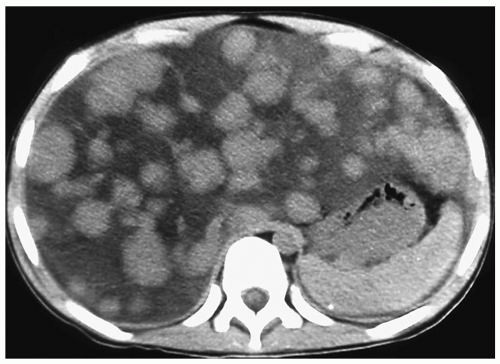
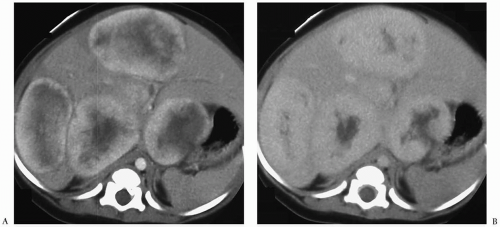
Hepatic metastases appear hypo- or hyperechoic relative to normal liver on sonography (Fig. 5.7), isoattenuating on contrast-enhanced CT (Fig. 5.8), hypointense on T1-weighted MR images, and hyperintense on T2-weighted and fat-suppressed MR images. Nearly all hepatic metastases in children are hypovascular and appear hypoattenuating or hypointense during the portal venous phase of enhancement. They may show rim enhancement during the hepatic arterial phase. Other findings include mass effect with displacement of vessels and vessel invasion or amputation.
Hepatic metastases in children are typically hypovascular.
Diffuse parenchymal replacement by tumor is typical of stage IV-S neuroblastoma. All imaging examinations show widespread heterogeneity (Fig. 5.9). The differential diagnoses for this imaging appearance include diffusely infiltrative hepatoblastoma and cirrhosis, most commonly secondary to tyrosinemia.
Diffuse parenchymal replacement is typical of stage IV-S neuroblastoma.
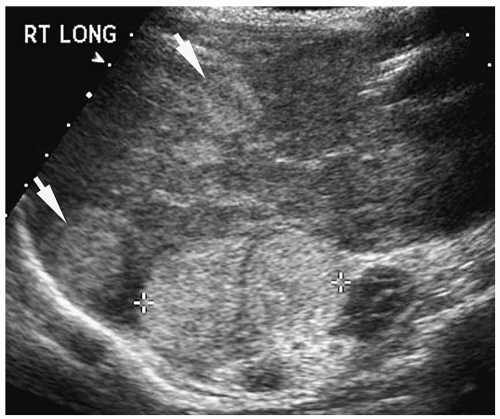 Figure 5.7 Metastatic neuroblastoma. Longitudinal sonogram shows two hyperechoic masses (arrows) within the right lobe, as well as the primary adrenal mass (cursors). |
Benign Neoplasms
Hemangioendothelioma.
Hemangioendothelioma is the most common benign hepatic tumor in children. Most patients are infants who present with congestive heart failure due to high-output overcirculation. Occasionally, affected patients present with bleeding diathesis secondary to platelet sequestration (Kasabach-Merritt syndrome) or massive hemoperitoneum due to spontaneous tumor rupture. Pathologically, hemangioendothelioma contains vascular channels lined by plump endothelial cells and enclosed within a reticular network (1).
Hemangioendothelioma is the most common benign hepatic tumor in children.
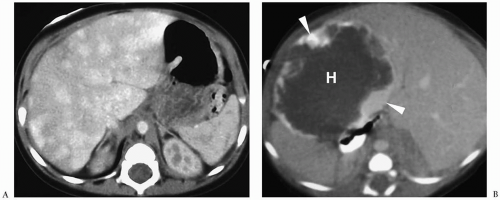
Hemangioendothelioma can be a solitary or multifocal lesion (15). On sonography, it may be hyper- or hypoechoic relative to adjacent liver (Fig. 5.10). Color Doppler shows peritumoral or intratumoral flow or a mixed pattern and high-frequency peak systolic shifts and diminished systolic-diastolic flow variation. On noncontrast-enhanced CT scans and T1-and T2-weighted MR images, the attenuation and signal intensity, respectively, of the tumor is similar to that of blood vessels (Fig. 5.11). Areas of fibrosis, necrosis, or hemorrhage that
are frequently present within larger lesions may cause a heterogeneous appearance. Hemangioendotheliomas typically show a distinctive pattern of enhancement after administration of iodinated contrast or gadolinium, characterized by early peripheral nodular enhancement with complete central opacification on delayed images (16) (Fig. 5.12). However, small tumors may show early uniform fill-in without centripetal opacification, whereas large lesions may demonstrate persistent central hypoattenuation or hypointensity due to areas of fibrosis or thrombosis (Fig. 5.13). Other findings include a small aorta distal to the
level of the celiac artery and a relatively larger suprahepatic aorta, related to shunting of a large part of the cardiac output into the tumor via the celiac artery.
are frequently present within larger lesions may cause a heterogeneous appearance. Hemangioendotheliomas typically show a distinctive pattern of enhancement after administration of iodinated contrast or gadolinium, characterized by early peripheral nodular enhancement with complete central opacification on delayed images (16) (Fig. 5.12). However, small tumors may show early uniform fill-in without centripetal opacification, whereas large lesions may demonstrate persistent central hypoattenuation or hypointensity due to areas of fibrosis or thrombosis (Fig. 5.13). Other findings include a small aorta distal to the
level of the celiac artery and a relatively larger suprahepatic aorta, related to shunting of a large part of the cardiac output into the tumor via the celiac artery.
Hemangioendotheliomas show early peripheral enhancement with central opacification on delayed images.
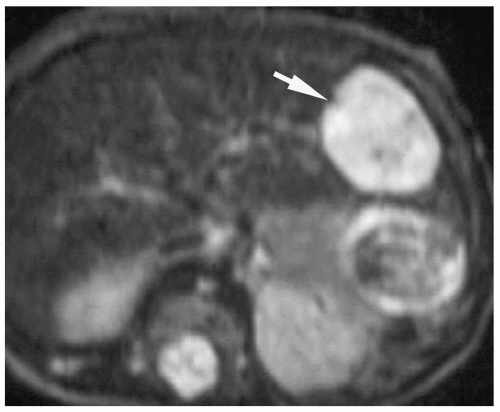 Figure 5.11 Hemangioendothelioma. T2-weighted MR image shows a high signal intensity lesion (arrow) in the left lobe of the liver. |
Because hemangioendotheliomas have a natural history of regression and involution within 12 to 18 months, the initial treatment is medical management, including digitalis, diuretics, steroids, and interferon. If these methods fail, chemotherapy, irradiation, or embolic or surgical treatment may be required for treatment.
Hemangioendotheliomas are usually managed medically.
Hemangioma.
Cavernous hemangioma is rare in childhood and usually affects older children and adolescents. It is usually asymptomatic and detected as an incidental finding on sonography or CT. Pathologically, cavernous hemangiomas contain multiple small, blood-filled spaces that are lined by mature, flat endothelial cells and separated by fibrous septa.
Cavernous hemangioma affects older children and adolescents.
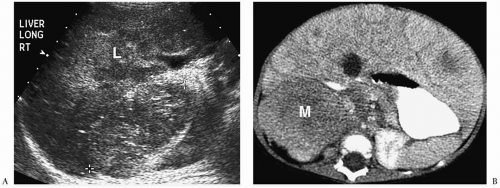
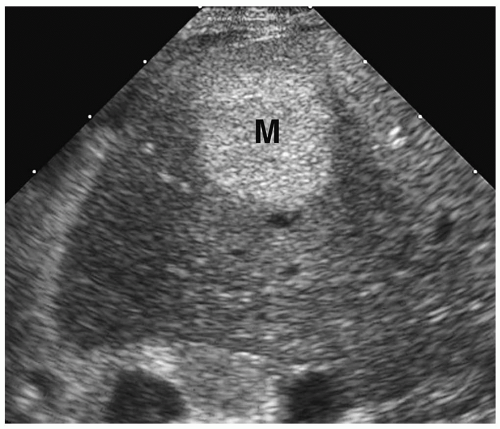
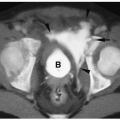
The classic sonographic appearance of hepatic hemangioma is that of a hyperechoic, homogeneous mass with well-defined margins (Fig. 5.14). The CT and MRI appearances of hemangioma are similar to those of hemangioendotheliomas.
Mesenchymal Hamartoma.
Mesenchymal hamartoma is a benign tumor that is thought to be a congenital abnormality originating in the connective tissue along the portal tracts. On pathologic section, it is a well-circumscribed mass composed of multiple cystic spaces of varying size, which are separated by fibrous septations. It usually affects children 2 to 3 years of age and is slightly more common in boys than girls. Affected patients present with an asymptomatic abdominal mass or abdominal distention. Malignant transformation to mesenchymal hamartoma has been reported but is rare.
Mesenchymal hamartoma is a benign mass composed of multiple cystic spaces separated by fibrous septations.
On all imaging studies, mesenchymal hamartoma usually appears as a multilocular mass containing fluid-filled locules and thin intermixed septa (Fig. 5.15). The cystic locules are anechoic or hypoechoic on sonography, low attenuation on CT, low signal intensity on T1-weighted sequences, and high signal intensity on T2-weighted and fat-suppressed sequences. However, the appearance may vary if the fluid contents are highly proteinaceous or contain debris. The solid portions of the mass can enhance after the administration of CT or MRI contrast agents.
Epithelial Lesions.
Focal nodular hyperplasia (FNH) and hepatic adenomas account for less than 5% of hepatic tumors in children. FNH is composed of normal hepatocytes, bile ducts, and Kupffer cells (17). A central stellate-shaped scar, containing bile ducts and arteries, is a typical feature, as is a subcapsular location. There is no strong association with pre-existing abnormalities. The tumor is usually asymptomatic and discovered incidentally on imaging studies.
Focal nodular hyperplasia (FNH) and hepatic adenomas account for less than 5% of hepatic tumors in children.
Hepatic adenomas consist of normal hepatocytes. Bile ducts and portal tracts are absent. Hepatic adenomas in childhood have been associated with type I glycogen storage disease (von Gierke disease), Fanconi anemia, and galactosemia. Patients may be asymptomatic or they may present with hepatomegaly or abdominal pain due to spontaneous hemorrhage.
On sonography, unenhanced CT, and T1-weighted MR, FNH often has imaging features similar to that of hepatic parenchyma. Occasionally, it may be slightly echogenic, hypoattenuating, or hypointense relative to normal parenchyma. On T2-weighted and fat-suppressed images, it may be isointense or hyperintense to hepatic parenchyma. On CT and MRI, FNH shows intense enhancement in the arterial phase of imaging (Fig. 5.16). During the portal venous phase, FNH becomes hypoattenuating or hypointense relative to normal hepatic parenchyma. The central scar is hypoattenuating on noncontrast CT, hypointense on T1-weighted images, and hyperintense on T2-weighted images. It does not enhance during the hepatic arterial phase, but it may show delayed enhancement in the portal venous phase. By comparison, the central scar in fibrolamellar carcinoma is hypointense on T2-weighted images and shows no enhancement after intravenous administration of CT or MR contrast agents.
FNH shows intense enhancement in the arterial phase.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree