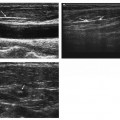
1 Liver Biopsy Liver biopsies are classified into random liver biopsies and target-specific liver lesion biopsies. A random liver biopsy is a liver tissue sample that is obtained to evaluate diffuse liver disease such as There are two types of random liver biopsies (Table 1.1): Fig. 1.1 Single fluoroscopic image in the first step of a transjugular liver biopsy. The first step is selective catheterization of one of the hepatic veins. The right hepatic vein is preferable because it provides the greater wire and catheter purchase. (A) On a frontal projection the right hepatic vein usually starts (its caval orifice: arrowhead) near the vertebro-phrenic angle, which is close to the junction. A 5-French catheter has been passed down the superior vena cava, through the right atrium (RA) and into the inferior vena cava (IVC). A right turn is made and the catheter is passed all the way down near the periphery (subhepatic capsule: arrow). (B) Single fluoroscopic image, which is a magnified view of the periphery of the liver at the end of the 5-French catheter seen in Fig. 1.1A. Contrast is injected very gently through the catheter tip (arrow), which stains the hepatic parenchyma (arrowheads). This proves that the catheter is wedged and a wedged hepatic pressure can be obtained. The result obtained when the wedged hepatic pressure is subtracted from the central venous pressure (CVP) is helpful in determining the porto-systemic gradient to help diagnose portal hypertension. (C) Single fluoroscopic image where a 0.035-inch wire is passed down the catheter (arrow). The 5-French catheter has been removed and a curved-tip metal introducer sheath is being passed down the wire. The tip of the curved-tip introducer sheath is at the arrowhead. (D) Single fluoroscopic image that is a magnified view of the periphery of the liver. The curved-tip metal introducer sheath tip had been advanced down the wire into the periphery of the liver (arrow). A 20-gauge Trucut needle is advanced coaxially through the metal curved-tip introducer sheath (housed within sheath). The sheath directs the needle to the desired location (hepatic periphery) without injuring the structures it passes (cava, right atrium, hepatic veins). As can be seen the needle is tip-to-tip with the sheath. (E) Single fluoroscopic image that is a magnified view of the periphery of the liver. The 20-gauge Trucut needle is advanced coaxially through the metal sheath. The Trucut needle has a groove along its side (between arrows). The hepatic parenchyma falls into it. (F) Single fluoroscopic image that is a magnified view of the periphery of the liver. The 20-gauge Trucut needle has been “fired.” The outer metal covering (arrowheads) quickly passes over the needle groove and “shaves” off the hepatic parenchyma, which had fallen into the groove (between arrows). The shaved hepatic parenchyma now housed in the needle groove is the 20-gauge core sample. A liver lesion biopsy is a liver tissue sample that is obtained to evaluate a specific focal liver lesion such as Fig. 1.2 Planning ultrasound-guided biopsy approach based on computed tomography (CT) findings. (A) Contrast-enhanced axial CT image at the level of the porta hepatis. The image demonstrates an easily accessible right hepatic lobe from intercostal approaches (arrows) and an easily accessible left hepatic lobe from a subxyphoid (epigastric) approach (arrowheads). (B) Contrast-enhanced axial CT image at the level of the aortic hiatus. The image demonstrates a small left hepatic lobe, which is still accessible from a subxyphoid (epigastric) approach (arrowheads). The purpose of this image is to compare with the prior image (Fig. 1.2A) the different sizes and configurations of left hepatic lobes. (C) Contrast-enhanced axial CT image at the level of the aortic hiatus. The patient is status post right hepatic lobectomy with resultant left hepatic compensatory hypertrophy. The image demonstrates a large left hepatic lobe (L), which is still accessible from a subxyphoid (epigastric) approach (arrowheads). An intercostal approach is not feasible. There is no right hepatic lobe. The place of the right hepatic lobe is occupied by the right hemicolon (C). (D) Unenhanced axial CT image at the level of the aortic hiatus. The patient has not had any liver surgery. The right hemicolon (C), however, rides high and occupies the right upper quadrant anteriorly. The image demonstrates that an intercostal approach is feasible (arrows) only posterior to the midaxillary line (dashed line). A focused ultrasound posterior to the midaxillary line should be performed to find the target liver. (E) Unenhanced axial CT image at the level of the porta hepatis. The patient has not had any liver surgery. The right hemicolon (C), however, rides high and occupies the right upper quadrant posteriorly between the kidney and the right hepatic lobe. The image demonstrates that an intercostal approach is feasible (arrows) only anterior to the midaxillary line (dashed line). (K, kidney; R, right hepatic lobe; L, left hepatic lobe; Sp, spleen; C, colon; S, stomach; A, aorta; I, inferior vena cava; Lu, lung) Fig. 1.3 (A) Preliver biopsy examination gray-scale ultrasound exam (transverse to the abdomen in the epigastric region) as a 21-gauge lidocaine needle is advanced to locally anesthetize the subcutaneous tissue and Glisson’s capsule. The lidocaine needle tip (arrow) is at the liver (Glisson’s) capsule. (B) Gray-scale ultrasound exam (transverse to the abdomen in the epigastric region). The operator has just “panned” the transducer cephalad. Right above the left liver lobe (L) sits the base of the heart. The image shows the left hepatic lobe between the transducer and the heart, which is seen in its short axis. (L, liver; RV, right ventricle; LV, left ventricle) Laboratory value evaluation mostly revolves around ruling out coagulopathy.
Classification and Indications
Random Liver Biopsy
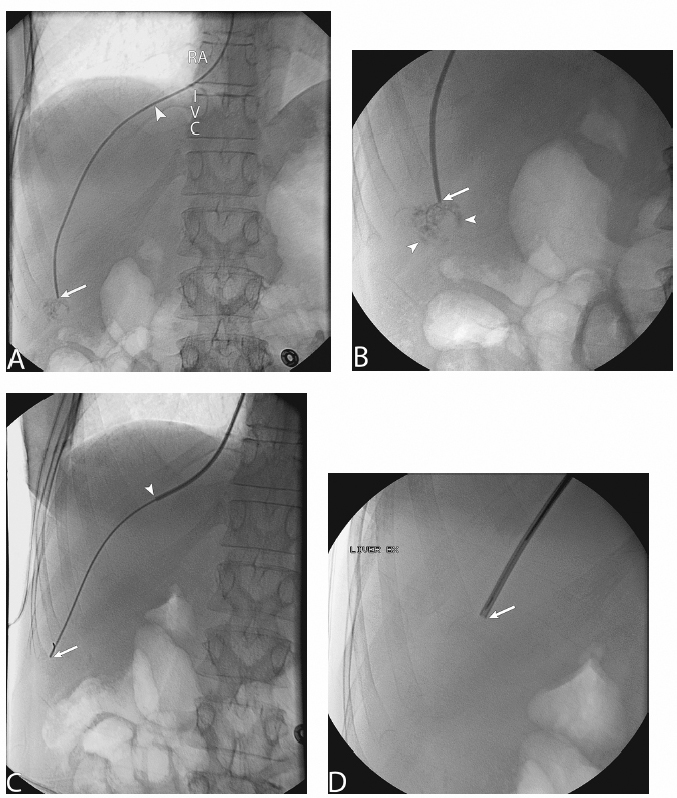
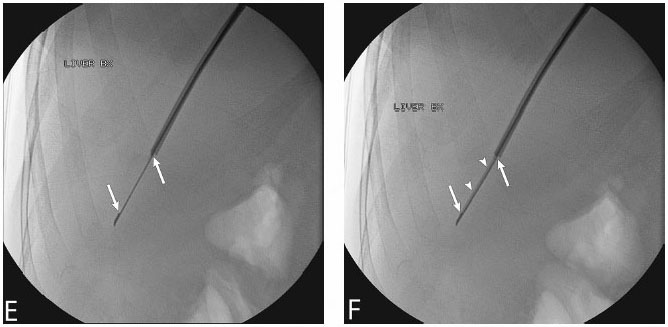
Target-selected Liver Biopsy/Liver Lesion Biopsy
Contraindications
Absolute Contraindications
Relative Contraindications
Preprocedural Evaluation
Evaluate Prior Cross-sectional Imaging
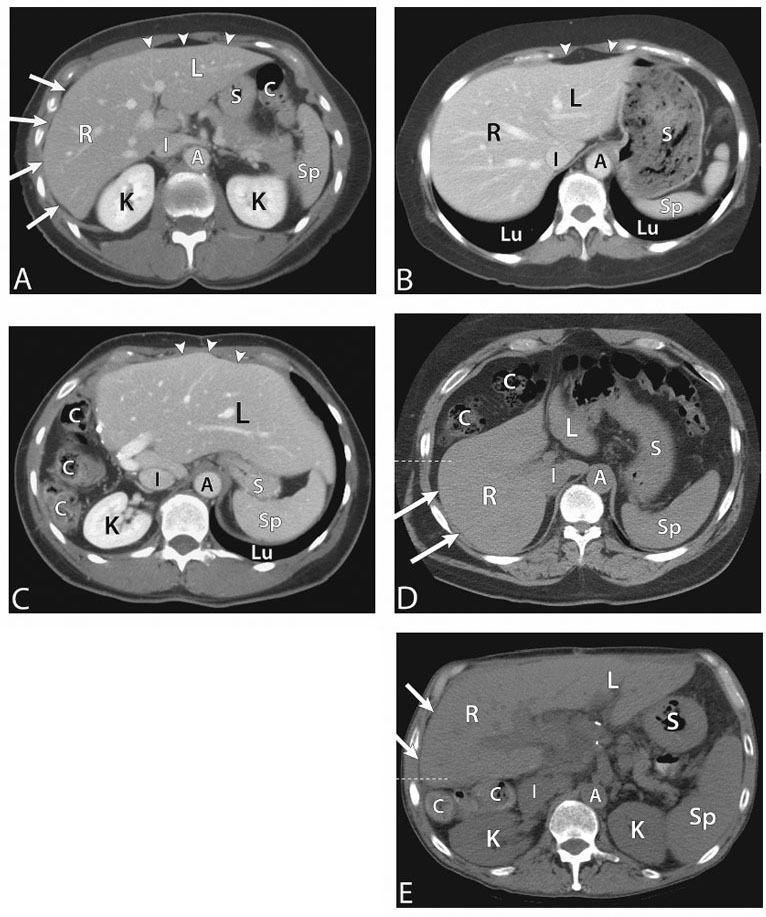
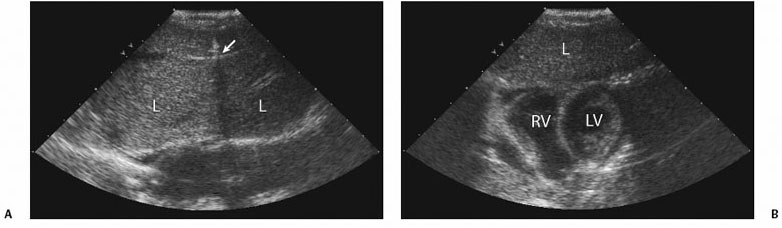
Evaluate Prebiopsy Laboratory Values
Obtain Informed Consent
Equipment
Ultrasound Guidance
Standard Surgical Preparation and Draping
Local Infiltrative Analgesia Administration
Sharp Access and Biopsy
Technique
Intravenous Access
Prebiopsy Ultrasound Examination
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree