The RTOG, EORTC, and SWOG (IJROBP 2011) presented an atlas for the contouring of organs at risk for thoracic radiotherapy. (PMID 20934273)
New Target Delineation Contours
FIGURE 13-12. Stereotactic body radiotherapy treatment (SBRT) planning for centrally located tumors. With 4D CT scans, a uniform 0.5 cm expansion in all directions to the ITV (yellow) will define the PTV (red).
1. ANATOMY
1.1. Gross Anatomy
• The right lung has three lobes as a result of a second fissure, the horizontal fissure, which separates the middle lobe from the upper lobe and extends from the anterior margin into the oblique fissure.1
• The left lung is composed of two lobes: an upper and lower lobe. The lingular portion of the left upper lobe corresponds to the middle lobe on the right.
• The trachea enters the superior mediastinum and bifurcates at the carina approximately at the level of the fifth thoracic vertebra (Fig. 13-1A).2
• The hila contain the bronchi, pulmonary arteries and veins, various branches from the pulmonary plexus, bronchial arteries and veins, and lymphatics.
• The proximal bronchial tree consist of the carina, right and left main bronchi, right and left upper lobe bronchi, intermedius bronchus, right middle lobe bronchus, lingular bronchus, right and left lower lobe bronchi (Fig. 13-1B). The zone of the proximal bronchial tree is defined as a volume of 2 cm in all directions around these structures.
1.2. Lung Lymph Node Stations
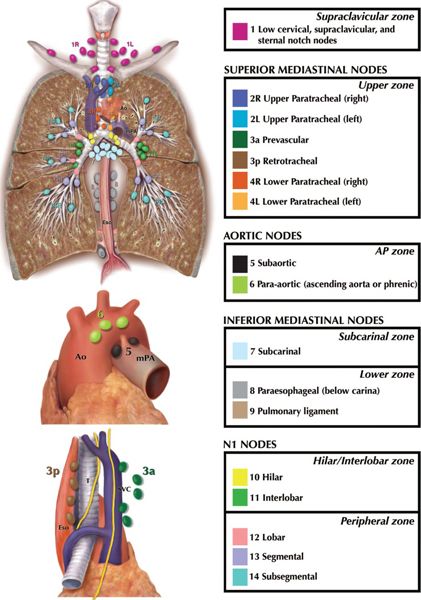
• Figures 13-2 and 13-3 show the 2009 International Association for the Study of Lung Cancer (IASLC) lymph node map corresponding to the 7th edition of the American Joint Committee on Cancer (AJCC) TNM staging manual.3 Anatomical boundaries of each nodal station are detailed below.
• Supraclavicular Zone
º 1. Low cervical, supraclavicular and sternal notch nodes. Upper border: lower margin of cricoid cartilage. Lower border: clavicles bilaterally and, in the midline, the upper border of the manubrium. For lymph node station 1, the midline of the trachea serves as the border between 1R and 1L.
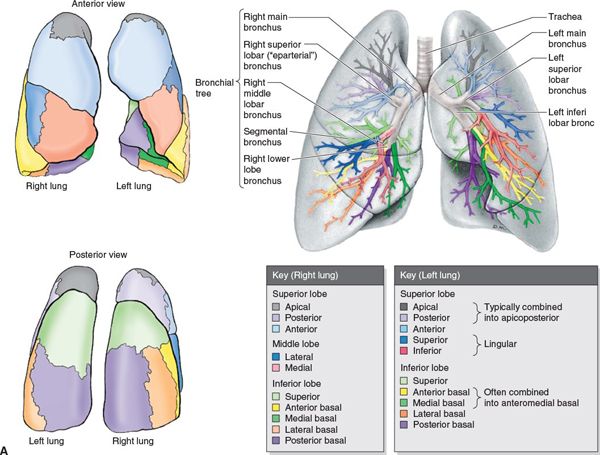
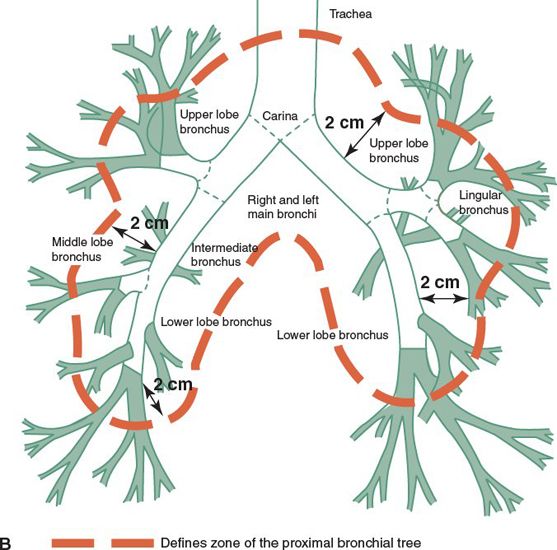
FIGURE 13-1. (A) Segmental anatomy of the lungs. (From Moore KL, Dalley AF. Clinically Oriented Anatomy, 4th ed. Baltimore, MD: Lippincott Williams & Wilkins, 1999.)
• Superior Mediastinal Nodes
º 2R. Upper paratracheal nodes. Upper border: apex of the right lung and in the midline, the upper border of the manubrium. Lower border: intersection of caudal margin of innominate vein with the trachea. As with lymph node station 4R, 2R includes nodes extending to the left lateral border of the trachea.
º 2L. Upper border: apex of the left lung, and in the midline, the upper border of the manubrium. Lower border: superior border of the aortic arch.
º 3a. Prevascular nodes. On the right: Upper border: apex of chest. Lower border: level of carina. Anterior border: posterior aspect of sternum. Posterior border: anterior border of superior vena cava. On the left: Upper border: apex of chest. Lower border: level of carina. Anterior border: posterior aspect of sternum. Posterior border: left carotid artery.
º 3p. Retrotracheal nodes. Located posterior to trachea. Upper border: apex of chest
Lower border: carina.
º 4R. Lower paratracheal nodes. Includes right paratracheal nodes, and pretracheal nodes extending to the left lateral border of trachea. Upper border: intersection of caudal margin of innominate vein with the trachea. Lower border: lower border of azygos vein.
º 4L. Includes nodes to the left of the left lateral border of the trachea, medial to the ligamentum arteriosum. Upper border: upper margin of the aortic arch. Lower border: upper rim of the left main pulmonary artery.
• Aortic Nodes
º 5. Subaortic nodes. Lateral to the ligamentum arteriosum. Upper border: the lower border of the aortic arch. Lower border: upper rim of the left main pulmonary artery.
º 6. Para-aortic nodes. Anterior and lateral to the ascending aorta and aortic arch. Upper border: a line tangential to the upper border of the aortic arch. Lower border: the lower border of the aortic arch.
• Inferior Mediastinal Nodes
º 7. Subcarinal nodes. Upper border: the carina of the trachea. Lower border: the upper border of the lower lobe bronchus on the left; the lower border of the bronchus intermedius on the right.
º 8. Paraesophageal nodes (below carina). Nodes lying adjacent to the wall of the esophagus and to the right or left of the midline, excluding subcarinal nodes. Upper border: the upper border of the lower lobe bronchus on the left; the lower border of the bronchus intermedius on the right. Lower border: the diaphragm.
º 9. Pulmonary ligament nodes. Nodes lying within the pulmonary ligament. Upper border: the inferior pulmonary vein. Lower border: the diaphragm.
FIGURE 13-2. Regional nodal stations for lung cancer. The International Association for the Study of Lung Cancer (IASLC) lymph node map includes the proposed grouping of lymph node stations into “zones” for the purposes of prognostic analyses. (Reprinted from: Goldstraw P (ed.) IASLC Staging Manual in Thoracic Oncology. Orange Park, FL: Editorial Rx Press, 2009, with permission. This figure is also reprinted in the Journal of Thoracic Oncology; see reference 68.)
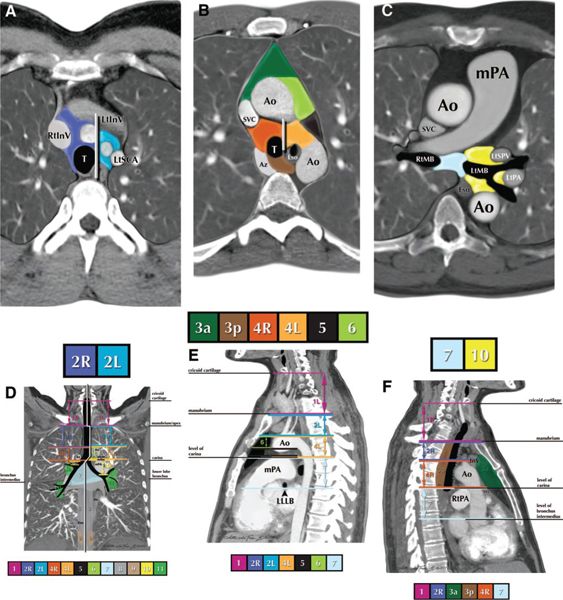
FIGURE 13-3. Nodal station boundaries for lung cancer. A–F: Illustrations of how the IASLC lymph node map can be applied to clinical staging by computed tomography (CT) scan in axial (A–C), coronal (D), and sagittal (E, F) views. The border between the right and left paratracheal region is shown in (A) and (B). Ao, aorta; AV, azygos vein; Br, bronchus; IA, innominate artery; IV, innominate vein; LA, ligamentum arteriosum; LIV, left innominate vein; LSA, left subclavian artery; PA, pulmonary artery; PV, pulmonary vein; RIV, right innominate vein; SVC, superior vena cava. (Reprinted from: Goldstraw P (ed.) IASLC Staging Manual in Thoracic Oncology. Orange Park, FL: Editorial Rx Press, 2009, with permission. This figure is also reprinted in the Journal of Thoracic Oncology; see reference 68.)
• N1 Nodes
º 10. Hilar nodes. Include nodes immediately adjacent to the mainstem bronchus and hilar vessels including the proximal portions of the pulmonary veins and main pulmonary artery. Upper border: the lower rim of the azygos vein on the right; upper rim of the pulmonary artery on the left. Lower border: interlobar region bilaterally.
º 11. Interlobar nodes. Between the origin of the lobar bronchi.
º 12. Lobar nodes. Adjacent to the lobar bronchi.
º 13. Segmental nodes. Adjacent to the segmental bronchi.
º 14. Subsegmental nodes. Adjacent to the subsegmental bronchi.
1.3. Lymphatic Drainage
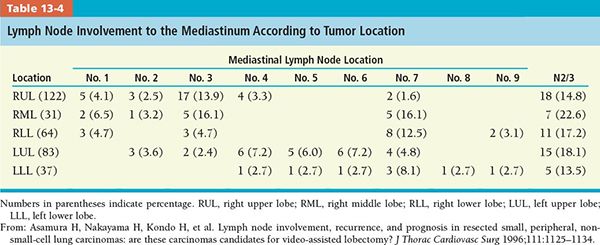
• The drainage for each pulmonary lobe is shown in Figure 13-4.4
• Upper lobes drainage: Right upper lobe lymphatics drain to the right paratracheal lymph nodes (4R, 2R). Left upper lobe lymphatics drain more frequently into the para-aortic and subaortic lymph nodes (5, 6, and 4L), but also to the venous angle of the opposite superior mediastinum.
• Middle and lower lobes drainage: The right and left lower lobe lymphatics drain into the hilar and the subcarinal nodes (7) and from there to the right paratracheal nodes (4R, 2R). The left lower lobe also may drain into the left paratracheal nodes (4L, 2L).
• Skip metastases: Direct tumor drainage to the mediastinal lymph nodes bypassing the hilar and interlobar nodes can be seen clinically in 7% to 26% of resected lung cancer specimens. They are more common in upper lobe lesions.
• In the case of chest wall involvement, there is a risk of spread to the intercostal nodes located close to the intercostal vessels and nerves. Paravertebral nodes situated either lateral to or in front of the vertebral bodies may be located along the pathway of the intercostal lymph collector.
2. NATURAL HISTORY
• Lung cancer is the leading cause of cancer death in the United States and the rest of the world.5–8 Unfortunately, most patients already have locally advanced lung cancer at the time of diagnosis.
• Small-cell lung cancers (SCLC) have a higher incidence of distant metastases than non-small-cell lung cancers (NSCLC); of the latter, adenocarcinoma has the highest potential for distant metastases.
FIGURE 13-4. Standard pattern of lymphatic drainage based on 192 lymphoscintigraphies carried out in 179 patients without known nodal dissemination. For the right lobes, most of the lymph flowed into the right supraclavicular nodes through the subcarinal or right tracheobronchial (hilar and paratracheal) nodes. In contrast, the lymphatic drainage from the left lung was more variable: the lymph can reach both the left and right supraclavicular lymph nodes, especially in the case of the lower lobe. (Redrawn from Hata E, Hayakawa K, Miyamoto H, et al. Rationale for extended lymphadenectomy for lung cancer. Theor Surg 1990;5:19–27, with permission.)
• Squamous cell carcinoma tends to spread more locally or intrathoracically rather than hematogenously.
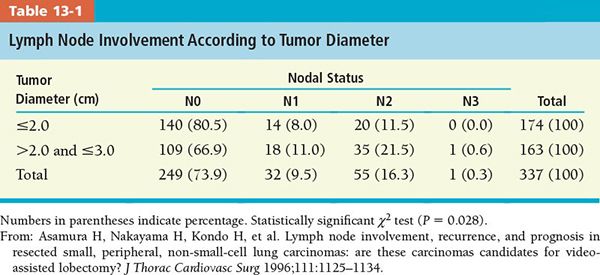
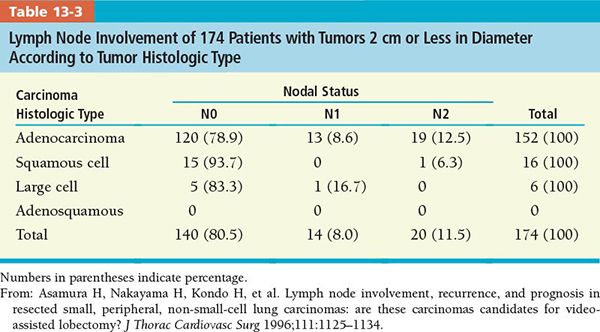
• Tables 13-1 and 13-2 present lymph node involvement by tumor diameter and histologic type, respectively.
3. DIAGNOSIS AND STAGING SYSTEM
3.1. Signs and Symptoms
• The majority of patients present with symptomatic disease.
• Cough (75%), hemoptysis (57%), dyspnea, and recurrent pneumonia are common major symptoms resulting from airway obstruction by central bronchogenic lesions.
• Patients with peripheral tumors are frequently asymptomatic unless there is involvement of the chest wall or intercostal nerves. Dyspnea and chest pain is also common when tumors invade the pleura, chest wall, or mediastinal structures.
• Hoarseness may result from recurrent laryngeal nerve involvement by mediastinal metastasis (more common with left lung tumors and with aorticopulmonary window lymphadenopathy).
• Dysphagia may be caused by compression of the esophagus by tumor.
• Superior vena cava syndrome can arise with right lung tumors or right mediastinal nodal metastasis.
• Tumors at the lung apex may involve the cervical or thoracic nerves, resulting in Pancoast syndrome or superior sulcus tumor syndrome (Horner syndrome, brachial plexopathy, and shoulder pain). Sympathetic nerve involvement results in Horner syndrome (enophthalmos, ptosis, meiosis, and ipsilateral loss of sweating).
• Involvement of the phrenic nerve can result in dyspnea and paralysis of the hemidiaphragm.

• CNS symptoms are seen with brain metastasis or paraneoplastic disease, such as hyponatremia or hypercalcemia (SCLC).
3.2. Physical Examination
• Signs of partial or complete bronchial obstruction, pleural effusion, pneumonia, or atelectasis may be detected by chest examination.
• Inspection of the head and neck may reveal regional lymphatic (N3) spread.
• Metastatic disease can be found by examination of the abdomen (liver metastasis: hepatomegaly) and neuroaxis (cerebral and/or spinal cord metastasis).
3.3. Imaging
3.3.1. Computed Tomography
• Routine chest x-ray is the most common radiologic examination.
• Computed tomography (CT) has been the principal diagnostic tool for diagnosing primary lesions, and the radiological detection of lymph node metastases is based on nodal enlargement, but it cannot differentiate inflammatory disease from neoplasia.
• Mediastinal nodes less than 1 cm in diameter are considered unlikely to contain metastatic disease. Nodes 1 to 2 cm are intermediate, and those larger than 2 cm in a patient with bronchogenic carcinoma almost certainly are metastatic.9
• A convenient and reasonably easy approach is to use a cutoff of 1 cm in the short-axis diameter.10 In a study by McLoud et al.,11 the rate of positive mediastinal lymph node involvement increased from 13% when the node measured less than 1 cm to 62% for nodes of 2 to 2.9 cm.
• CT specificity according to mediastinal nodal site varied from 72% for station 10R (right hilar) to 94% for station 10L (left hilar) when the criteria for positivity were 1 cm or more.11
• In the presence of obstructive pneumonitis, the rate of false positive lymph node involvement increased to 45%.
• If nodes in the draining territory of the tumor are enlarged (more than 1 cm in the short axis) and at least 0.5 cm larger than the nodes in the nondraining territories, CT specificity improves with a positive predictive value of 95%.12
3.3.2. Positron Emission Tomography/Computed Tomography
• Positron emission tomography (PET) scanning with 18F-fluorodeoxyglucose (18F-FDG) is routinely used to determine the malignant nature of suspicious lesions, to more accurately define tumor extent, including lymph node involvement,13–16 and to aid in treatment planning.17 In one study, PET influenced radiation delivery in 65% patients receiving definitive radiotherapy (RT).18
• Sensitivity and specificity for staging of individual patients range from approximately 60% to more than 90%, particularly if the PET scans were read in conjunction with the CT scans.19
• A high negative predictive value (~95%) of PET for mediastinal lymph node metastasis implies that invasive procedures are probably not necessary in patients with negative findings of PET in the mediastinum.
• However, 74% positive predictive value means that patients will need pathologic confirmation when a mediastinal hot spot is found on PET.20
3.4. Staging
• The current staging system is the 7th edition of AJCC.3 There are a number of changes in the T classifications, such as (a) division of T1 into T1a (<2 cm) and T1b (2 to 3 cm); (b) division of T2 into T2a (3 to 5 cm) and T2b (5 to 7 cm); and (c) reclassification of T2 tumors >7 cm as T3. Readers are strongly urged to consult the new AJCC staging manual.
• The location of the lymph nodes has been described according to the 2009 IASLC international lymph node map, which defines the nodal stations in relation to fixed anatomic structures, allowing a correlation between imaging studies and at endoscopy and surgery (Fig. 13-3; see also Fig. 13-4).
• Classically, SCLCs are generally staged more simply as limited (confined to one hemithorax/encompassed by a single radiation portal) or extensive. As of 7th edition of AJCC, the TMN classification is also recommended for SCLCs and carcinoid tumors of the lung.
3.5. Detection of Nodal Metastasis
• Cervical mediastinoscopy (through a supra-sternal approach) can biopsy mediastinal lymph node involvement in the left and right upper paratracheal nodes (stations 2L and 2R), left and right lower paratracheal nodes (stations 4L and 4R), and the anterior portion of the subcarinal nodes (station 7).
• Anterior mediastinotomy/Chamberlain procedure is used to biopsy the subaortic and para-aortic nodes (stations 5 and 6), which may be involved with left upper lobe lesions.
• Bronchoscopic ultrasound with fine-needle aspiration can biopsy nodes accessed from the tracheobronchial tree and can readily sample nodal stations 1, 2, 3, 4, 7, 10, and 11.
• Endoscopic ultrasound (EUS) with fine-needle aspiration can be performed on all the mediastinal nodes that can be accessed from the esophagus. EUS particularly provides access to nodes in the lower mediastinum (stations 7, 8, and 9).
• Asamura et al.21 analyzed involvement of the lymph nodes in 337 patients according to tumor size, histologic type, and location (Tables 13-1 to 13-4). Eighty-eight patients (26.1%) had lymph node involvement: 32 (9.5%) at N1, 55 (16.3%) at N2, and 1 patient at N3.
• According to this study, complete hilar and mediastinal lymph node dissection should be done routinely at resection because of the relatively high prevalence of the lymph node involvement, especially with adenocarcinoma lesions of diameter 2 cm or larger. However, for squamous cell carcinomas, the rate of mediastinal lymph node involvement is very low if the tumor is less than 2 cm in diameter.
4. GENERAL MANAGEMENT
4.1. Resectable Tumors
• If the disease is not initially surgically resectable, patients can be given combined modality treatment, such as neoadjuvant chemotherapy or chemoradiotherapy (CRT) followed by surgery with or without postoperative thoracic RT.
4.1.1. Preoperative Radiation, Chemotherapy, or Chemoradiation
• Several collaborative studies failed to show significant improvement in survival with use of preoperative radiation.22
• Preoperative chemotherapy has been associated with improved survival versus surgery alone for patients with N2-positive disease.23,24
• Trimodality preoperative CRT for N2-positive patients proved to be an encouraging approach in the SWOG 8805 trial.25
• INT 0139/Radiation Therapy Oncology Group (RTOG) 9309 trial demonstrated that preoperative chemoradiation to 45 Gy followed by surgery is associated with improved progression-free survival versus definitive chemoradiation to 61 Gy (5-year: 22% vs 11%) and better 5-year overall survival; pN0 disease was associated with improved survival, indicating the prognostic importance of mediastinal sterilization by preoperative chemoradiation; trimodality therapy may not be appropriate if pneumonectomy is required, due to a high rate of treatment-related deaths.26
• High-dose thoracic RT (approximately 60 Gy or higher) as a component of preoperative chemoradiation can be delivered safely and offers favorable outcomes.27–29
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree