Volume
Description
Gross tumor volume (GTV)
Pre-chemotherapy/surgery GTV (GTVp)
Contains gross tumor volume as identified on diagnostic imaging prior to chemotherapy and/or surgery
Post-chemotherapy/surgery GTV (GTVr)
Contains gross tumor volume as identified on diagnostic and/or planning imaging after management with chemotherapy and/or surgery
No prior treatment GTV (GTV)
Contains gross tumor volume identified on diagnostic and/or planning imaging in the absence of prior chemotherapy or surgery (e.g., in the case of primary or salvage RT)
Clinical target volume (CTV)
Contains entire post-chemotherapy/surgery or “no prior treatment” GTV
Generally contains pre-chemotherapy/surgery GTV (unless volume is unable to be safely treating in its entirety)
Excludes extent of pre-chemotherapy/surgery GTV that displaced normal, uninvolved tissue (bone, organs, muscles, etc.) prior to chemotherapy or surgery
Includes consideration of the following:
Image accuracy and quality. CTV should be increased in size to account for uncertainties such as suboptimal fusion of pre- and post-chemotherapy/surgery images to planning images or in the absence of pre-chemotherapy/surgery images
Changes in volume since the time of imaging
Pattern of disease spread
Potential subclinical involvement. Consider including nodes of unknown status near the site of disease, particularly if the questionable nodes belong to a nodal chain or group already partially encompassed by the CTV
Adjacent organ dose constraints
Can contain separate nodal volumes if ≤5 cm apart. Lesions >5 cm apart are treated with separate CTVs
Internal target volume (ITV)
Contains the CTV plus an internal margin that accounts for variation in CTV shape, size, and position (e.g., target movement with respiration)
Most relevant for targets within the chest and abdomen; may be unnecessary for other sites
Planning target volume (PTV)
Contains CTV or ITV plus a margin to account for setup uncertainties associated with patient position and beam alignment
Note that CTV to PTV expansions are patient- and institution-specific; any recommendations in this chapter or elsewhere must be adjusted to reflect factors unique to the patient and institution
Organs at risk (OAR)
Includes uninvolved normal structures at risk of RT-related toxicity for which RT planning or dose may be altered
2 Background
Non-Hodgkin lymphoma (NHL) is a heterogeneous group of B-cell, T-cell, and natural killer (NK)-cell neoplasms that lack the pathologic characteristics seen in Hodgkin disease (HD).
The World Health Organization (WHO) has classified approximately 80 unique forms of NHL (Swerdlow et al. 2008). These are traditionally grouped by tumor aggressiveness into indolent, aggressive, and highly aggressive NHL categories, as described in Table 2.
Indolent
Aggressive
Highly aggressive
Follicular lymphoma (grades I and II)
Follicular lymphoma (grade III)
Burkitt’s lymphoma
Marginal zone B-cell lymphoma: extra-nodal (MALT lymphoma), nodal (monocytoid), splenic
Diffuse large B-cell lymphoma (DLBCL)
Precursor B-cell or T-cell lymphoblastic lymphoma/leukemia
Small lymphocytic lymphoma
Mantle cell lymphoma
T-cell granular lymphocytic lymphoma
Anaplastic large cell lymphoma
Peripheral T-cell lymphoma
Angiocentric T-cell lymphoma
Angioimmunoblastic T-cell lymphoma
NHL can be further divided into nodal versus extra-nodal lymphomas. Although treatment of both nodal and extra-nodal NHL depends on tumor aggressiveness, extent of disease, and response to systemic therapies, management of extra-nodal lymphoma with radiotherapy (RT) is more clearly driven by site-specific considerations.
2.1 Evolution of Radiation Fields for Lymphoma
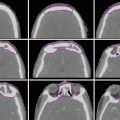
Historically, HD and NHL were managed with RT alone using extended fields such as mantle and inverted Y configurations, with higher rates of late toxicity associated with larger field size (Fig. 1a, b).

Fig. 1
Historical RT fields. Digitally reconstructed radiographs (DRR) of (a) mantle and (b) inverted Y extended fields and (c) axilla involved field RT
By the 1990s, combined modality therapy became a mainstay of lymphoma management. With the addition of increasingly successful chemotherapies and advancements in target localization such as 3D simulation, RT fields have become progressively smaller (Fig. 1).
Until recently, the standard of care for RT in both HD and NHL was involved field radiotherapy (IFRT). This began to change in 2006, when EORTC-GELA Lymphoma Group published guidelines for involved nodal radiotherapy (INRT) for HD (Girinsky et al. 2006) (Fig. 1c).
Unlike traditional IFRT fields that treat adjacent uninvolved lymph nodes, INRT limits treatment to only pre- and post-chemotherapy involved nodal volumes plus margin. Whereas IFRT traditionally uses bony landmarks to identify field borders, INRT volumes are delineated using definitions of gross tumor volume (GTV), clinical target volume (CTV), and planning target volume (PTV) per the International Commission on Radiation Units and Measurements (ICRU) Report 83 (Hodapp 2012).
However, INRT requires precisely fused pre- and post-chemotherapy imaging performed in the treatment position, which is often unattainable in clinical practice. To address this limitation, the International Lymphoma Radiation Oncology Group (ILROG) published consensus guidelines for the use of involved site radiotherapy (ISRT) for HD (Specht et al. 2013). ISRT targets the sites of pre- and post-chemotherapy disease involvement but also offers less stringent treatment volume definitions than ISRT to allow for uncertainties associated with less than optimal imaging.
ILROG has recently published consensus guidelines for the use of ISRT for nodal NHL(reference below), with similar guidelines pending for extra-nodal NHL (Illidge 2014). Although ISRT for NHL has not yet been validated through randomized trials, single-arm and retrospective data suggest comparable control with such reduced field sizes (Campbell et al. 2010; Zhang et al. 2012; Yu et al. 2010).
As ISRT is evolving to be the standard of care for RT in NHL, this chapter will focus on ISRT for both nodal and extra-nodal lymphomas. For recommendations regarding involved fields, reference should be made to Yahalom and Mauch’s guidelines for IFRT in HD (Yahalom and Mauch 2002).
2.2 General Principles of Involved Site Radiotherapy
ISRT requires 3D simulation with CT, PET-CT, or MRI. Pre-chemotherapy or pre-surgery diagnostic imaging is strongly recommended, particularly PET-CT with IV contrast as well as PO contrast for abdominopelvic sites. Post-chemotherapy PET-CT is also useful for identifying areas of residual FDG avidity.
These studies should be fused to simulation imaging when feasible. Although not a requirement for ISRT, diagnostic imaging should be performed in the treatment position whenever possible to optimize fusion.
Simulation and immobilization will be discussed by treatment site.
3 Nodal NHL
3.1 Principles of Management
In nodal NHL, RT may be indicated as primary therapy; consolidation following chemotherapy for bulky, residual, or unfavorable disease; or salvage for chemotherapy-refractory or recurrent disease.
RT dose varies by tumor aggressive, extent of disease and chemotherapy response.
See Table 3 for management strategies of nodal B-cell NHL (Illidge 2014).
Table 3
Management of nodal B-cell NHL
Aggressiveness
Stagea or clinical status
Treatment modality
RT dose (1.8–2 Gy fraction)
Target delineation considerations
Indolent B cell
Stages I–II
RT alone
24–30 Gy
CTV should generously cover adjacent nodes when RT is used as definitive therapy (e.g., CTV of at least 1–2 cm depending on the clinical scenario)
Stages III–IV
Rituximab, palliative RT, or observation
4–30 Gy
–
Aggressive B cell
Stages I–II and favorable
Stages I–II and unfavorable
Stages III–IV
R-CHOP × 3–4 cycles + RT; R-CHOP × 6–8 cycles
R-CHOP × 6–8 cycles +/− RT for sites of bulky disease or partial response
R-CHOP × 6–8 cycles +/− RT for sites of bulky disease or partial response
30–36 Gy
30–40 Gy
30–40 Gy
For planned combined chemoRT, CTV includes entire pre-chemotherapy GTV (minus uninvolved displaced tissues)
For RT to sites of residual disease, the treatment volume can be limited to sites with residual PET-avidity on post-chemotherapy imaging
Highly aggressive
–
Chemotherapy alone, with palliative RT to symptomatic sites
4–30 Gy
–
3.2 General Considerations for Cervical/Supraclavicular Nodal ISRT
The patient should be simulated in the supine position with hyperextension of the neck and the use of a long thermoplastic mask extending to the shoulder region for immobilization.
3D simulation should be performed with IV contrast.
GTV to CTV margin should be at least 1 cm. If applicable, the final CTV should also encompass the pre-chemotherapy/surgery GTV extent, excluding normal, uninvolved tissue that was displaced by the tumor prior to chemotherapy or surgery.
Relevant OARs include the cervical spinal cord, brachial plexus, and salivary glands.
For treatment with AP/PA technique:
Get Clinical Tree app for offline access
A posterior cervical cord block can be placed if cord dose exceeds 36 Gy.
A posterior mouth block is recommended to inhibit divergence through the mouth.
A laryngeal block can be added after 18 Gy or a 50% partial transmission laryngeal block can be used for the full duration of treatment unless prohibited by the presence of medial cervical lymph nodes (Fig.2).



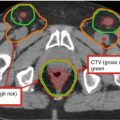
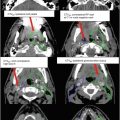
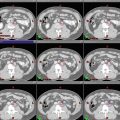
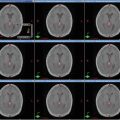
Fig. 2
(a–c) Cervical nodal ISRT planning images for a patient with stage IA diffuse large B-cell lymphoma with near-complete response after three cycles of R-CHOP. (a) The pre-chemotherapy FDG-avid GTV is delineated in purple. Also noted is benign physiologic pharyngeal uptake (white arrows). There were no other areas of FDG-avid disease. (b) Axial CT slices from the post-chemotherapy planning CT (left column) and the pre-chemotherapy PET-CT (right column) are fused to create a pre-chemotherapy GTV (orange), post-chemotherapy GTV (red), and CTV (green). Fusion of the two image sets is limited by differences in neck extension and arm position; this is particularly noticeable when locating the region of the pre-chemotherapy right level IV lymph node on the post-chemotherapy planning CT (red arrows). To account for greater uncertainty in target localization, the CTV in the corresponding regions is larger than the typical 1 cm CTV expansion. (c) post-chemotherapy GTV (red), CTV (green), and PTV (blue) are shown in on (1) coronal, (2) sagittal, and (3) axial slices. In this case, the GTV to CTV expansion is at least 1 cm, and the CTV to PTV expansion is 0.5 cm. Note that CTV to PTV margins depend on patient- and institution-specific factors, and therefore, universal recommendations for appropriate expansions cannot be provided
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree