(1)
Department of Radiation Oncology, Memorial Sloan Kettering Cancer Center, New York, NY
(2)
Department of Radiation Oncology, University of Toronto/Sunnybrook Health Sciences Centre, Toronto, ON
Abstract
The oropharynx is contiguous with the oral cavity anteriorly, the larynx and hypopharynx inferiorly, and the nasopharynx superiorly. It is commonly divided into four subsites: the tonsillar region, base of the tongue, soft palate, and pharyngeal wall
1 Anatomy and Patterns of Spread
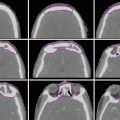
The oropharynx is contiguous with the oral cavity anteriorly, the larynx and hypopharynx inferiorly, and the nasopharynx superiorly. It is commonly divided into four subsites: the tonsillar region, base of the tongue, soft palate, and pharyngeal wall (Fig. 1).

Fig. 1
Subsites of the oropharynx: a tonsil, b base of the tongue, c soft palate, d pharyngeal wall
The anterior and posterior tonsillar pillars are mucosal folds produced by the underlying palatoglossal muscle and palatopharyngeal muscle, respectively. These pillars define the borders of the tonsillar fossa, which contains the palatine tonsil. Tonsillar cancers most commonly arise on the anterior pillar and may extend superiorly along this structure towards the soft palate or inferiorly towards the base of the tongue. Anterolateral spread along the pharyngeal constrictor muscle to the pterygomandibular raphe and retromolar trigone may occur. Superolateral spread to the infratemporal space can be seen in advanced cases.
The base of the tongue is bounded anteriorly by the circumvallate papillae. Inferiorly, the vallecula is considered part of the base of the tongue, while the epiglottis belongs to the supraglottic larynx. Cancers of the tongue base may spread anteriorly into the oral tongue and/or floor of the mouth or posteriorly/inferiorly via the vallecula into the preepiglottic space.
The inferior surface of the soft palate and uvula belong to the oropharynx, while the superior surface is a nasopharyngeal structure. Tumors of the soft palate may spread laterally/inferiorly to the tonsil via the anterior tonsillar pillar or superiorly towards the nasopharynx.
The superior pharyngeal constrictor muscle forms the posterior and lateral walls of the oropharynx. Cancers of the lateral and posterior pharyngeal walls may spread via the mucosa or submucosa towards the hypopharynx and/or nasopharynx. Skip lesions are not uncommon.

Fig. 2
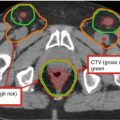
Estimated risk of pathologic nodal involvement by level when negative by CT imaging for T1–T2 oropharyngeal primary tumors (Sanguineti et al. 2009) (Figure printed with permission from publisher- Elsevier)
Lymphatic spread is predictable. Ipsilateral level II is the most common location for lymph node metastasis. Next echelon lymphatic drainage includes levels III and IV and the retropharyngeal lymph nodes. Involvement levels I and V is uncommon (Fig. 2).
The incidence of retropharyngeal nodal involvement varies by subsite. Bussels et al. reported a significantly higher incidence in those with primary tumors of the posterior pharyngeal wall (38 %) and soft palate (44 %) than those with primary tumors of the base of the tongue (13 %) or tonsil (14 %) (Bussels et al. 2006).
2 Diagnostic Workup Relevant for Target Volume Delineation
Both physical examination and imaging should be used for delineation of the gross tumor volume.
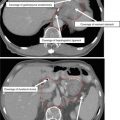
Visual inspection, palpation, and fiber-optic examination are critical for accurate delineation of mucosal extension. The true extent of mucosal extension may be missed on imaging but appreciated on clinical examination (Fig. 3).

Fig. 3
Direct visualization of anterior mucosal spread to retromolar trigone
MRI has distinct advantages in soft tissue discrimination and reduced dental amalgam interference. It provides complementary information to CT and PET and may allow for improved GTV and normal tissue delineation.
T2-weighted with fat saturation: assessment of retropharyngeal lymph nodes, parapharyngeal space, and preepiglottic space
T1-weighted pre-contrast: used primarily for evaluation of fate planes, especially parapharyngeal fat space for asymmetry. Additional utility for assessment of bone marrow
T1-weighted post-contrast: assessment of perineural invasion
The use of MRI requires co-registration or fusion with the CT simulation scan. The use of immobilization mask during MRI allows for improved fusion accuracy but may preclude the use of a dedicated head and neck coil.
Diffusion-weighted (DWI) MRI provides apparent diffusion coefficients (ADC), which are inversely correlated with tissue cellularity. DW-MRI has a high negative predictive value for the assessment of lymph node metastases (Vandecaveye et al. 2009).
CT remains superior to MRI for the assessment of cortical bone invasion. Administration of iodinated contrast is recommended for enhanced tumor discrimination.
FDG-PET provides metabolic information that is complementary to CT and MRI. It has been shown to reduce interobserver variability in tumor delineation and may aid in the identification of tumor extension missed by CT or MRI (Syed et al. 2005).
FDG-PET has been shown to provide metabolic information that is of prognostic value independent of tumor size and T-category (Romesser et al. 2012).
Limitations of FDG-PET include poor spatial resolution and low sensitivity for small-volume lymph node metastases. The absence of FDG uptake in an otherwise suspicious lymph node should not be considered reassuring.
3 Simulation and Daily Localization
The patient should be set up in the supine position with the neck extended. The immobilization device (Aquaplast mask) should provide adequate shoulder immobilization. Bite-block and/or mouth guard may be inserted.
CT simulation using 2.5–3 mm slice thickness with IV contrast. Isocenter is typically placed just above the arytenoid cartilages.
MRI and PET images, if available, may then be registered or fused to the CT simulation scan.
At MSKCC, image guidance is achieved with daily linear-accelerator-mounted 2D kV imaging and weekly kV conebeam CT. Alternative methods for image guidance include orthogonal kV imaging (“ExacTrac”) or linear-accelerator-mounted MV CT images (“TomoTherapy”).
CTV to PTV expansion of 3–5 mm, depending on the accuracy of daily patient positioning and image guidance.
4 Target Volume Delineation and Treatment Planning
4.1 Selected IMRT Dose Fractionation Schemes for Oropharyngeal Cancer
Standard fractionation (33 fractions) with integrated boost. Gross disease dose: 69.96 Gy in 33 fractions. High-risk subclinical disease dose: 59.4 Gy in 33 fractions. Low-risk subclinical disease dose: 54.12 Gy in 33 fractions. Single phase (simultaneous integrated boost), with 5 fractions weekly (Garden et al. 2013; Setton et al. 2012).
Standard fractionation (35 fractions) with cone-down. Gross disease dose: 70 Gy in 35 fractions. Subclinical disease dose: 54 Gy in 30 fractions. Initial phase (simultaneous integrated boost): 2 Gy per fraction to gross disease, 1.8 Gy per fraction to subclinical disease (30 fractions). Boost: 2 Gy per fraction to gross disease.
RTOG 00–22. Gross disease dose: 66 Gy in 30 fractions. Subclinical disease dose: 54 Gy in 30 fractions. Optional high-risk subclinical disease dose: 60 Gy in 30 fractions. Single phase with one plan (simultaneous integrated boost), with 5 fractions weekly (Eisbruch et al. 2010).
RTOG 10–16. Gross disease dose: 70 Gy in 35 fractions. High-risk subclinical disease dose: 56 Gy in 35 fractions. Low-risk subclinical disease dose: 50–52.5 Gy in 35 fractions. LAN field dose: 44 Gy in 22 fractions. Single phase with one plan (simultaneous integrated boost), six fractions weekly.
Concomitant boost (RTOG 90–03, 01–29): two phases, twice daily treatment for the last 12 days. Total duration of 6 weeks. 54 Gy in 30 fractions to gross and subclinical disease. For the last 12 fractions, a second daily fraction to gross disease of 1.5 Gy is delivered (at least 6-h interval) (Ang et al. 2010; Beitler et al. 2014).
4.2 Split-Field Versus Whole-Field IMRT
For patients without low neck disease, a low anterior AP field with larynx block is matched to the IMRT field at the isocenter just above the arytenoids. Dose is 45–50 Gy in 20–25 fractions, prescribed to 3 cm depth. In cases of gross involvement of the low neck or near the match-line, whole neck IMRT is preferred.
4.3 Suggested Target Volumes
Table 1
Suggested target volumes for gross disease
Target volumes | Definition and description |
|---|---|
GTV70 | Primary: all gross disease as defined by clinical examination (e.g., base of tongue tumors that are superficial and not apparent on imaging) and imaging |
Neck nodes: all suspicious (>1 cm, necrotic, enhancing, or FDG avid) lymph nodes. Borderline suspicious lymph nodes may be treated to an intermediate dose (66 Gy in 33 fractions) | |
CTV70 | Typically same as GTV70 (no added margin). Margin of 5 mm may be added if there is uncertainty in extent of gross tumor so that GTV70 + 5 mm = CTV70
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|