Location of primary tumor
Draining lymphatics
Distal anal canal, perianal skin, and anal verge
Superficial inguinal lymph nodes
Femoral nodes
External iliac nodes
Anal canal just proximal to dentate line
Internal pudendal
Hypogastric
Obturator nodes
Inferior and middle hemorrhoidal
Proximal anal canal
Perirectal
Superior hemorrhoidal
Nearly 20 % of patients present with involved nodes at the time of diagnosis.
2 Diagnostic Workup Relevant for Target Volume Delineation
Physical examination is an important part of the staging and planning process and should include detailed assessment of the characteristics of the primary tumor (size, anal sphincter competence, invasion of adjacent structures) as well as an assessment of inguinal lymph nodes.
Lymph nodes in the inguinal region that are suspicious but borderline should be biopsied (nearly 50 % of suspicious nodes are related to reactive hyperplasia).
Standard imaging includes CT or MRI of the pelvis to assess the primary tumor and status of the regional lymph nodes. CT of the chest and abdomen completes the metastatic workup.
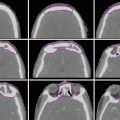
These tumors can be well visualized on PET, so PET/CT is becoming a standard part of staging and recommended for planning to help delineate extent of gross disease (Fig. 1).

Fig. 1
An example of how PET can help delineate GTV. The GTV (red) is seen on representative axial, sagittal, and coronal images, respectively, on both the treatment planning CT and PET in the upper panels. Additional representative axial slices are shown below in the lower panels
Areas of low uptake on PET should not supersede physical exam findings or abnormalities seen on CT.
3 Simulation and Daily Localization
The patient can be simulated in the supine position in a body mold or other immobilization device to ensure reproducibility. Prone position with the use of a belly board can be used to allow for anterior displacement of the bowel, but setup reproducibility is more variable and using bolus or additional electron fields to supplement dose to the inguinal regions would not be possible. A radiopaque marker should be placed on the anus.
CT simulation using ≤3 mm thickness with IV contrast should be performed to delineate the pelvic blood vessels and gross tumor volume (GTV). If PET/CT is available, a PET/CT fusion should be obtained to aid in target volume delineation. MRI may also be useful.
Bladder filling/emptying should be considered. A full bladder may keep bowel from migrating into the pelvis. An empty bladder may be more reproducible.
We recommend daily orthogonal kilovoltage images and weekly cone beam CT scans (to assess soft tissue) to verify alignment during treatment. Cone beam CTs may be done more frequently if there is significant variation in bladder and/or rectal filling.
4 Target Volume Delineation and Treatment Planning
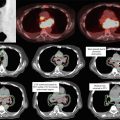
Conventional radiation therapy for anal canal cancers is complex due to the need to irradiate the groins as well as the pelvis. The “thunderbird” technique was the most common method used to treat anal cancer. An example of the thunderbird technique compared to an IMRT plan is shown in Fig. 2. In the thunderbird technique, a wide AP field is used to encompass the inguinal lymph nodes. The PA field is narrowed to exclude the inguinal lymph nodes so that the femoral heads are also excluded. An additional boost field must therefore be used to supplement dose to the inguinal regions. This can be accomplished with either photons (with a skin match or a deep match) or electrons. A more detailed description of these thunderbird technique variations is described by Gilroy et al. (2004). Figure 2 shows a standard dose distribution of a photon/electron thunderbird technique.

Fig. 2
Comparison of a photon/electron thunderbird technique (panel a and b) and intensity-modulated radiotherapy (panel c and d)
RTOG 0529 (Kachnic et al. 2013) has established the feasibility of IMRT in a multi-institution setting and demonstrated lower rates of grade 2 or higher dermatologic toxicity and lower rates of grade 3 or higher gastrointestinal or genitourinary toxicity when compared to historical controls in the RTOG 98-11 (Ajani et al. 2008) trial.
The primary gross tumor volume (GTV-P) is defined as all gross disease on physical examination and imaging.
The nodal GTV (GTV-N) includes all nodes that are ≥1.5 cm, PET positive, or biopsy proven. In the absence of biopsy, any clinically or radiographically suspicious lymph nodes should be included in the GTV-N.
The high-risk clinical target volume (CTV-HR) should include the entire mesorectum, the bilateral internal iliac nodes inferior to the inferior-most level of the sacroiliac joint, and the inguinal or external iliac lymphatics if the inguinal nodes are involved. Additional details are given in Table 2.
Table 2
Suggested target volumes for gross and microscopic disease
Target volumes
Definition and description
GTV (gross tumor volume)
Primary (GTV-P): all gross disease on physical examination and imaging
Regional nodes (GTV-N): all nodes ≥1.5 cm, PET-positive, or biopsy proven include any lymph node in doubt as GTV in the absence of a biopsy
Clinical target volume (CTV) gross disease
CTV-P should cover the GTV-P with 1.5–2.5 cm margin expansion but excluding uninvolved bone, muscle, or air. The CTV-N should cover the GTV-N with a 1.0–1.5 cm margin but excluding uninvolved bone, muscle or air
CTV-high risk (CTV-HR)
Should cover the entire mesorectum, the right and left internal iliac lymph nodes inferior to the inferior-most level of the sacroiliac joint, and the inguinal or external iliac lymphatics if the inguinal nodes are uninvolved
A 1.8-cm wide volume between the external and internal iliac vessels is needed to cover the obturator nodes (Taylor et al. 2005)
CTV-low risk (CTV-LR)
Should include the uninvolved inguinal, external iliac, and internal iliac nodes superior to the inferior-most level of the sacroiliac joint
A 1.8-cm wide volume between the external and internal iliac vessels is needed to cover the obturator nodes (Taylor et al. 2005)
Planning target volume (PTV)
Each CTV should be expanded by 0.5–1 cm, depending on the physician’s comfort level with setup accuracy, frequency of imaging, and the use of IGRT
The low-risk CTV (CTV-LR) should include the uninvolved inguinal, external iliac, and internal iliac nodes superior to the inferiormost level of the sacroiliac joint.
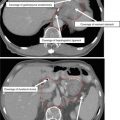
Figure 3 shows a case example of a T2N0 anal cancer.


Fig. 3
(a) A patient with T2N0 anal canal cancer. This patient was simulated supine using PET/CT simulation with a 2.5 mm thickness on each slice. CTV is shown. Note that these are representative slices and not all slices are included. CTV (low risk, cyan), CTV (high risk, orange), CTV (gross disease, green), and GTV (red, shaded) are shown. (b) Enhanced view of the lower pelvis showing CTV (low risk, blue), CTV (high risk, orange), CTV (gross disease, green), and GTV (red, shaded)
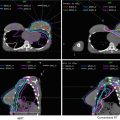
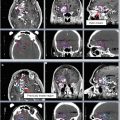
Figure 4 shows a case example of a T3N3 anal cancer.


Fig. 4
(a) A patient with T3N3 anal canal cancer with bilateral inguinal lymph node involvement. This patient was simulated supine using PET/CT simulation with a 2.5 mm thickness on each slice. CTV (low risk, cyan), CTV (high risk, orange), CTV (gross disease, green), and GTV (red, shaded) are shown. Note that these are representative slices and not all slices are included. (b) Enhanced view of the lower pelvis showing CTV (high risk, orange), CTV (gross disease, green), and GTV (red, shaded)
Detailed contouring atlases available include the RTOG anorectal contouring atlas (Myerson et al. 2009) and the Australasian GI Trials Group Atlas (Ng et al. 2012).
The RTOG anorectal contouring atlas (Myerson et al. 2009) describes 3 CTV regions that should be included for all patients with anal canal cancer. CTV-A includes the perirectal, presacral, and internal iliac regions. CTV-B includes the external iliac nodes. CTV-C includes the inguinal region. Table 3 provides a more detailed description of these regions.
Clinical target volume
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access