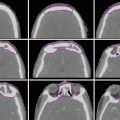
Fig. 1
Mountain-Dresler lymph node staging system (from Mountain and Dresler (1997), with permission)
2 Diagnostic Workup Relevant for Target Volume Delineation
Target volume delineation for NSCLC is dependent on the following studies: computed tomography (CT) scan of the chest with contrast, positron emission tomography (PET)/CT imaging, and, particularly in the case of NSCLC, comprehensive mediastinal evaluation, either with mediastinoscopy or endobronchial ultrasound (EBUS).
On CT scan with contrast, we consider lymph nodes measuring at least 1 cm in shortest diameter are to be positive radiographically for malignant involvement.
It has been found that with regard to tumor contouring, PET imaging provides novel information in up to 20 % of patients when compared to CT scan alone, and with upstaging occurring in approximately 15–30 % of patients (Nawara et al. 2012; Bradley et al. 2004), and that this modality can also be used to distinguish atelectasis from tumor.
PET/CT scan is used in combination for imaging delineation. PET/CT scan provides a high level of sensitivity, and CT scan with contrast can be used to delineate involved lymph nodes from surrounding vasculature.
PET/CT scans have been found to have false-positive rates of involved lymph nodes of up to 30 %, depending on the patient population (De Ruysscher et al. 2012; De Ruysscher 2011), and therefore, histologic confirmation for the purposes of target delineation should be obtained when possible, particularly if positivity will substantially affect the dose to normal structures (e.g., contralateral lymph nodes).
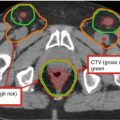
In addition, background avidity in normal thoracic tissues (lung, esophagus, aorta) can vary greatly between structures (Chen et al. 2013), indicating that this background uptake should also be taken into account with treatment planning (Fig. 2).

Fig. 2
PET/CT imaging of a locally advanced NSCLC. (a) CT imaging alone, (b) PET imaging alone, and (c, d) fused images
3 Simulation and Daily Localization
Patients are typically immobilized with their arms over their head to maximize the number of potential beam angles. The upper body cradle extends inferiorly to provide immobilization through the thorax.
CT simulations are performed with a slice thickness of 2.5–3 mm. Intravenous contrast can be considered when necessary to differentiate tumor involvement from mediastinal structures such as the vasculature.
Four-dimensional (4D) CT scans are acquired, to account for internal motion. When the magnitude of respiratory motion is ≥1 cm and regular, patients are treated with a “free-breathing” approach, in which they breathe regularly during treatment. If irregular or motion >1 cm, then respiratory management is considered, either deep breath hold (inspiratory or expiratory) or respiratory gating, in which radiation is delivered at specific periods of the breathing cycle. Both techniques have been shown to be beneficial in reducing target volumes for tumors that have substantial motion (Muirhead et al. 2010; Underberg et al. 2005).
For patients to tolerate the deep breath hold technique, they need to be able to maintain the appropriate position in the respiratory cycle for at least 15 s, which is difficult for a significant percentage of patients with lung cancer.
The 4D CT scan range typically extends from at least the thoracic inlet to the inferior portion of the diaphragm.
Image fusion with PET/CT scans is recommended when feasible, particularly in cases with atelectasis or when directly adjacent to critical structures. Our iCTV-to-PTV margin is 5 mm if daily kV imaging is used and 3 mm for daily CBCT.
Image registration and fusion applications with MRI and PET scans should be used to help in the delineation of target volumes, especially for regions of interest encompassing the GTV, skull base, brainstem, and optic chiasm. The GTV and CTV and normal tissues should be outlined on all CT slices in which the structures exist.
At our institution, we routinely utilize daily kV imaging and weekly CT scan alignment for localization (cone-beam CT scan or CT scan on rails). Daily CT scan localization is utilized when more precise localization is necessary (e.g., tumors adjacent to the spinal cord), when the size of the tumor is rapidly changing, or when bony landmarks are not representative of internal anatomy. Prior studies have shown that with daily kV imaging, interfractional variation is approximately 5 mm (Nelson et al. 2008) and, with CBCT, can be reduced to approximately 3 mm. These are thus the margins that are utilized at our institution (Borst et al. 2007).
4 Target Volume Delineation and Treatment Planning
When conformal techniques are utilized and the treating physician has performed a comprehensive evaluation of disease involvement, an involved field approach is used for target volume delineation in NSCLC, due to the published low likelihood of disease recurrence in elective lymph nodes (Rosenzweig et al. 2001, 2007). An involved field technique plus inclusion of the ipsilateral hilum is also now recommended in the Radiation Therapy Oncology Group’s (RTOG’s) recent randomized trial examining the effect of dose on survival (RTOG 0538).
Accounting for respiratory motion is critical in treatment planning. After assessing respiratory motion at the time of simulation, the standard International Commission of Radiological Units and Measurements (ICRU) volumes are as follows:
Gross tumor volume (GTV) – gross disease, including primary tumor and lymph nodes.
Clinical target volume (CTV) – gross disease + region at risk for microscopic spread. In the setting of NSCLC in which an involved nodal technique is used, this volume often includes the remainder of the involved lymph node station. For example, in the case of an involved subcarinal lymph node, the node would be covered in the iGTV and then expanded and the entire subcarinal nodal station also encompassed in the iCTV. This volume is also edited to respect anatomical boundaries (taken off of bones, arteries, etc.). Margins for microscopic disease of 0.5–1.0 cm for either microscopic disease are generally considered acceptable, though studies establishing the extent of microscopic spread beyond that visualized on CT scan have been published in the setting of NSCLC (Giraud et al. 2000) but not SCLC.
Internal target volume (ITV) – clinical target volume + respiratory motion.
Planning target volume (PTV) – ITV + daily setup variation. At our institution, we utilize daily imaging for all patients being treated with locally advanced disease.
At our institution, we frequently utilize a slight variation on standard treatment volumes, in which the GTV, rather than the CTV, is expanded to account for respiratory motion. One advantage to this technique from a practical standpoint is that it is often easier to assess motion on simulation imaging when evaluating the discrete tumor volume of the GTV if one exists. That is, when evaluating motion on the CTV, this region may consist of an anatomical space such as a lymph node region, leading to a less precise estimate of the true motion of the target volume. The target contours defined using this method are as follows:
GTV – gross disease
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree