This article discusses the use of MR imaging in various acute infectious diseases of the head and neck, with particular emphasis on situations where MR imaging provides additional information that can significantly impact treatment decisions and outcomes. MR imaging findings of various disease processes are discussed, based on the head and neck compartments from which they originate. Specifically, infectious entities of the orbit, paranasal sinuses, pharynx, oral cavity (including periodontal disease), salivary glands, temporal bone, and lymph nodes are described in detail.
Key points
- •
The severity of head and neck infections can often be ascertained from the clinical history and physical examination.
- •
In some sinus and periorbital infections, MR imaging is crucial for identifying spread of infection into the orbital and intracranial compartments and helps to guide management.
- •
MR imaging is unsurpassed in its ability to identify and evaluate complications of middle ear and temporal bone infections.
- •
MR imaging detects osteomyelitis and is useful to differentiate osteomyelitis from acute bone infarction in the setting of sickle cell disease.
- •
MR imaging is an invaluable tool to pinpoint the exact origin and extent of retropharyngeal and prevertebral infections and to identify possible associated complications.
Introduction
Acute head and neck infections can evolve as slowly progressive smoldering processes, or advance as rapidly debilitating entities with clinical consequences that can often be life threatening. The clinical presentation of the infectious process in question varies depending on the specific head and neck compartment that is primarily affected. The severity and aggressive nature of the disease process can usually be sufficiently elucidated though the clinical examination and history.
Imaging is often relied upon to assist in the evaluation of the anatomic extent of the acute infection and to identify any pertinent complications that could contribute to patient morbidity and mortality if left unidentified and untreated. Although computed tomography (CT) is the first-line modality in the acute setting of many uncomplicated infectious processes, MR imaging is the modality of choice in examining the exact scope of certain complicated infections owing to its superior delineation of soft tissue contrast, particularly when intracranial complications are suspected. MR imaging is unquestionably the modality of choice for high-resolution evaluation of multiple entities including facial neuritis, optic neuritis, labyrinthitis, petrous apicitis, and acute osteomyelitis. MR imaging is also a valuable tool to evaluate the bone marrow and differentiate between entities such as osteomyelitis and bone infarct. MR imaging also detects vascular complications caused by acute infections, such as sinus venous thrombosis. The ability to identify these dreaded complications and drastically influence clinical management confirms the utility of MR imaging in the evaluation of acute head and neck infections.
This article discusses the use of MR imaging in various acute infectious diseases of the head and neck, with particular emphasis on situations where MR imaging provides additional information that can significantly impact treatment decisions and outcomes. MR imaging findings of various disease processes are discussed, based on the head and neck compartments from which they originate. Specifically, infectious entities of the orbit, paranasal sinuses, pharynx, oral cavity (including periodontal disease), salivary glands, temporal bone, and lymph nodes are described in detail.
Introduction
Acute head and neck infections can evolve as slowly progressive smoldering processes, or advance as rapidly debilitating entities with clinical consequences that can often be life threatening. The clinical presentation of the infectious process in question varies depending on the specific head and neck compartment that is primarily affected. The severity and aggressive nature of the disease process can usually be sufficiently elucidated though the clinical examination and history.
Imaging is often relied upon to assist in the evaluation of the anatomic extent of the acute infection and to identify any pertinent complications that could contribute to patient morbidity and mortality if left unidentified and untreated. Although computed tomography (CT) is the first-line modality in the acute setting of many uncomplicated infectious processes, MR imaging is the modality of choice in examining the exact scope of certain complicated infections owing to its superior delineation of soft tissue contrast, particularly when intracranial complications are suspected. MR imaging is unquestionably the modality of choice for high-resolution evaluation of multiple entities including facial neuritis, optic neuritis, labyrinthitis, petrous apicitis, and acute osteomyelitis. MR imaging is also a valuable tool to evaluate the bone marrow and differentiate between entities such as osteomyelitis and bone infarct. MR imaging also detects vascular complications caused by acute infections, such as sinus venous thrombosis. The ability to identify these dreaded complications and drastically influence clinical management confirms the utility of MR imaging in the evaluation of acute head and neck infections.
This article discusses the use of MR imaging in various acute infectious diseases of the head and neck, with particular emphasis on situations where MR imaging provides additional information that can significantly impact treatment decisions and outcomes. MR imaging findings of various disease processes are discussed, based on the head and neck compartments from which they originate. Specifically, infectious entities of the orbit, paranasal sinuses, pharynx, oral cavity (including periodontal disease), salivary glands, temporal bone, and lymph nodes are described in detail.
Orbits
Periorbital and Orbital Infections
The orbital septum is the anatomic landmark that distinguishes the periorbital (preseptal) tissues from the orbit proper (postseptal tissues). The orbital septum is a thin membranous sheet extending from the orbital periosteum. Superiorly, it inserts and blends with the aponeurosis of the levator palpebrae superioris, and inferiorly it blends with the tarsal plates. This structure is not readily demonstrated on imaging, but it serves as a barrier to posterior spread of infections from the periorbital tissues into the orbit proper.
Periorbital cellulitis typically occurs secondary to a nearby sinonasal, facial, or dental infection that spreads to involve the periorbital tissues. Local trauma may also serve as an etiology. Typical presenting symptoms include pain, eyelid swelling and erythema, conjunctivitis, and fever. On MR imaging, periorbital cellulitis usually manifests as edema, soft tissue swelling, and diffuse periorbital soft tissue enhancement. A loculated abscess within the periorbital or surrounding facial soft tissues may also be present.
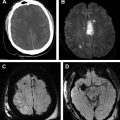
In contrast, orbital cellulitis usually results as a complication of adjacent paranasal sinusitis ( Fig. 1 ) and occasionally from a dental infection. Embedded posttraumatic foreign bodies can also contribute to this process. Typical presenting symptoms are similar to periorbital cellulitis, with the addition of proptosis as a predominant feature. Orbital cellulitis is associated with an increased risk of devastating neurologic sequelae, including ophthalmic vein thrombosis, venous sinus thrombosis, mycotic aneurysm, meningitis, and intracranial abscess. Thus, identifying an infection in the orbit is of paramount importance to help guide management and prevent poor outcomes.
The MR imaging findings of orbital cellulitis include fat stranding and rim-enhancing abscess formation, which can be intraconal or extraconal (often subperiosteal) and edema and abnormal enhancement of the extraocular muscles. Noninfectious conditions, including idiopathic orbital inflammatory syndrome (“pseudotumor”; Fig. 2 ), and immunoglobulin G4 (IgG4)-related disease can mimic this infection. Grave’s ophthalmopathy is also a differential consideration, but can be differentiated by noting the classic sparing of the tendinous insertions.
Treatment of orbital cellulitis typically involves hospital admission with administration of intravenous antibiotics and possible drainage of loculated orbital collections, if present. In contrast, uncomplicated periorbital cellulitis can be treated on an outpatient basis with oral antibiotics.
Of note, dacryocystitis is an infection that results from obstruction of the medial nasolacrimal duct that can be complicated by periorbital and, rarely, orbital cellulitis. Infection from Streptococcus pneumoniae accounts for almost 25% of the cases, although other microorganisms from the Streptococcus and Staphylococcus families can also be responsible. Typical clinical presentation involves focal swelling along the medial canthus, conjunctivitis, and purulent drainage. MR imaging may demonstrate a dilated fluid-filled lacrimal sac along the medial canthus with peripheral rim enhancement on postcontrast images. When clinical assessment is limited owing to concurrent periorbital and orbital cellulitis, the value of MR imaging is its ability to accurately depict these complications. Treatment may be either medical or surgical, depending on patient symptomatology and the presence of associated complications.
Paranasal sinuses
Bacterial Infection
A variety of organisms have been implicated as causes of acute and chronic sinusitis, including S pneumoniae and other Streptococcus strains, Staphylococcus aureus , Moraxella catarrhalis , Pseudomonas species, particularly in immunosuppressed and diabetics, and anaerobic bacteria. Differentiating between acute and chronic sinusitis can only be made by considering the timeframe during which it has been present. Inflammation of the paranasal sinus mucosa present for fewer than 4 weeks is defined as acute sinusitis, whereas chronic sinusitis refers to disease that is present for longer than 12 weeks. Both entities clinically present with nasal congestion, purulent discharge, headache, maxillary and dental pain, and reduced sense of taste or smell.
Imaging is not typically necessary to diagnose sinusitis, particularly in the acute phase. Although CT is the first-line modality, MR imaging can be performed when intracranial or intraorbital complications are suspected clinically. MR imaging is sensitive in identifying inflammation of the paranasal sinus mucosa. Although the diagnosis of sinusitis should not be based solely on imaging findings, greater than 4 mm of sinus mucosal thickening suggests a greater likelihood of sinusitis. Findings that increase the diagnostic confidence of acute sinusitis include the presence of air–fluid levels or complete fluid opacification of one or more of the paranasal sinuses. If there is an underlying obstructing tumor, fluid-sensitive sequences can be useful in demonstrating the relatively hypointense mass adjacent to hyperintense inflamed sinus mucosa and fluid. In the chronic stage of sinusitis, much of the sinus fluid becomes inspissated with an increase in protein concentration. As a result, fluid signal on T1-weighted images, which is initially hypointense, becomes hyperintense, and signal on T2-weighted images becomes progressively darker.
Sinus infection may result in orbital and intracranial complications owing to their proximity to the sinonasal cavities (see Fig. 1 ). Approximately 3% of patients with sinusitis (especially ethmoid sinusitis) can develop orbital complications, particularly in children or young adults. Orbital complications, including periorbital/orbital cellulitis or subperiosteal abscess formation, are well depicted on MR imaging (described in greater detail in the Orbits section). Orbital complications are much more common than intracranial complications.
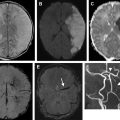
Neurologic complications, including meningitis, cavernous sinus thrombosis, and subperiosteal and intracranial abscesses, can also be readily identified with MR images. Pott’s puffy tumor is a complication of sinusitis characterized by subperiosteal abscess and osteomyelitis of the frontal bone, resulting from spread of infection beyond the confines of the sinus into the surrounding bone via infectious thrombophlebitis of penetrating valveless emissary veins. On clinical examination, this entity presents as a fluctuant mass overlying the eyebrow. MR imaging is not only useful in demonstrating the extent of osteomyelitis, subperiosteal abscess, and surrounding soft tissue inflammation, but more valuable in identifying intracranial complications such as intraaxial or extraaxial abscess ( Fig. 3 ), or venous sinus thrombosis.
Fungal Infection
Fungal sinusitis can be classified into two groups: noninvasive and invasive. The noninvasive category is further classified into allergic fungal sinusitis and mycetoma (fungus ball). Both subtypes of the noninvasive category are characterized by disease involvement that is limited to the lumen of the affected sinus without penetration past the mucosa, and is seen typically in immunocompetent patients.
Allergic fungal sinusitis is the most common form of fungal sinusitis, and typically occurs in younger patients with a history of atopy, asthma, nasal polyposis, and aspirin intolerance. Aspergillus species are commonly responsible for allergic fungal sinusitis. Patients present with complaints of chronic nasal congestion and headaches. Characteristic MR imaging findings ( Fig. 4 ) include near complete sinus opacification of multiple sinuses with T2-hypointense material owing to its low water and high protein content and the high concentration of paramagnetic substances.
Mycetoma is characterized by the presence of a fungus ball limited to the cavity of an affected sinus and is also most commonly secondary to Aspergillus species. Clinical symptoms are usually minimal. Characteristic MR imaging findings include an intraluminal mass that is usually T1 and T2 hypointense owing to lack of water content, with scattered areas of susceptibility artifact secondary to calcification and paramagnetic substances such as manganese, iron, and magnesium. Usually, a single sinus is involved, most commonly the maxillary sinus ( Fig. 5 ), followed by the sphenoid sinus.
Invasive fungal sinusitis is characterized by spread of disease beyond the mucosa, with invasion of penetrating vessels that allows the infection to infiltrate into the surrounding osseous structures, orbital, and intracranial compartments ( Fig. 6 ). Acute invasive fungal sinusitis is a rapidly progressive and deteriorating infection with a 50% to 80% fatality rate, most often afflicting immunocompromised and poorly controlled diabetic patients. Zygomycetes such as Rhizopus and Mucor are the organisms classically involved in this form of sinusitis. In addition to the usual symptoms of sinusitis, invasive fungal sinusitis often presents with neurologic symptoms including cranial nerve impairment, seizures, mental status changes, coma, proptosis, and visual field defects secondary to intracranial and orbital invasion. Chronic invasive fungal sinusitis differs from its acute counterpart in that it progresses slowly over the course of months to years. Characteristic MR imaging findings of invasive fungal sinusitis include unilateral erosive/destructive changes of the osseous walls of the affected sinuses with extension into the orbit and brain, resulting in orbital cellulitis, cranial nerve impairment, and cavernous sinus thrombosis. Internal carotid artery invasion may cause occlusion or pseudoaneurysm formation, with an associated increased risk of cerebral infarct and hemorrhage.
Salivary glands
Sialadenitis
Sialadenitis refers to infection or inflammation of the salivary glands ( Fig. 7 ). Viral sialadenitis, often secondary to mumps infection from paramyxovirus, is more common and typically presents as bilateral parotitis ( Fig. 8 ). Acute bacterial causes, most commonly Staphylococcus or Streptococcus species, usually present unilaterally and occur in elderly, debilitated, or postoperative dehydrated patients. Sialolithiasis is a major risk factor for development of bacterial sialadenitis ( Fig. 9 ), and most commonly affects the submandibular glands, possibly as result of increased viscosity of submandibular secretions. Thus, the submandibular glands are most commonly affected by bacterial sialadenitis. Oral cavity or floor of mouth malignancy may also cause outflow duct obstruction and associated inflammation of the affected salivary gland ( Fig. 10 ). Typical presentation involves swelling and pain or focal tenderness. In the case of bacterial sialadenitis associated with an obstructing sialolith, pain can often be exacerbated by activities that stimulate saliva production (“salivary colic”).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree