Neuroimaging plays a critical role in the management of patients with acute stroke syndrome, with diagnostic, therapeutic, and prognostic implications. A multiparametric magnetic resonance (MR) imaging protocol in the emergency setting can address both primary goals of neuroimaging (ie, detection of infarction and exclusion of hemorrhage) and secondary goals of neuroimaging (ie, identifying the site of arterial occlusion, tissue characterization for defining infarct core and penumbra, and determining stroke cause/mechanism). MR imaging provides accurate diagnosis of acute ischemic stroke (AIS) and can differentiate AIS from other potential differential diagnoses.
Key points
- •
Neuroimaging plays a critical role in the management of patients with acute stroke syndrome, with diagnostic, therapeutic, and prognostic implications.
- •
A multiparametric magnetic resonance (MR) imaging protocol in the emergency setting can address both primary goals of neuroimaging (ie, detection of infarction and exclusion of hemorrhage) and secondary goals of neuroimaging (ie, identifying the site of arterial occlusion, tissue characterization for defining infarct core and penumbra, and determining stroke cause/mechanism).
- •
MR imaging provides accurate diagnosis of acute ischemic stroke (AIS) and can differentiate AIS from other potential differential diagnoses.
Introduction
Stroke is a common and serious disorder, with an annual incidence of approximately 795,000 in the United States, of which approximately 85% are ischemic and 15% are hemorrhagic. Neuroimaging plays a critical role in the diagnosis and management of patients with acute stroke. The neuroimaging evaluation of patients with suspected acute stroke has significantly evolved over the past few decades from a simple noncontrast computed tomography (CT) to now frequently including multiparametric data, including vascular and perfusion imaging.
Although the time window of 3.0 to 4.5 hours still applies for intravenous–thrombolytic treatment, recent clinical trials with encouraging results on the potential role of endovascular treatment have created new possibilities for advanced stroke treatment, with the potential for treatment of many more patients beyond the 4.5 hours time window. This possibility makes the role of advanced imaging even more crucial, with the potential for expanding the treatment window for patients with acute stroke if carefully screened and selected based on appropriate imaging criteria. The paradigm is changing from “time is brain” to “imaging is brain.”
This article reviews the role of magnetic resonance (MR) imaging and multiparametric MR imaging for the evaluation of patients presenting with acute stroke syndrome, such as acute ischemic stroke (AIS), intracranial hemorrhage (ICH), and transient ischemic attack (TIA), and some of the potential challenging differential diagnoses in the acute emergency setting. It also reviews our institutional technical and clinical experience in MR imaging of patients with acute stroke and provides some clinical examples.
Introduction
Stroke is a common and serious disorder, with an annual incidence of approximately 795,000 in the United States, of which approximately 85% are ischemic and 15% are hemorrhagic. Neuroimaging plays a critical role in the diagnosis and management of patients with acute stroke. The neuroimaging evaluation of patients with suspected acute stroke has significantly evolved over the past few decades from a simple noncontrast computed tomography (CT) to now frequently including multiparametric data, including vascular and perfusion imaging.
Although the time window of 3.0 to 4.5 hours still applies for intravenous–thrombolytic treatment, recent clinical trials with encouraging results on the potential role of endovascular treatment have created new possibilities for advanced stroke treatment, with the potential for treatment of many more patients beyond the 4.5 hours time window. This possibility makes the role of advanced imaging even more crucial, with the potential for expanding the treatment window for patients with acute stroke if carefully screened and selected based on appropriate imaging criteria. The paradigm is changing from “time is brain” to “imaging is brain.”
This article reviews the role of magnetic resonance (MR) imaging and multiparametric MR imaging for the evaluation of patients presenting with acute stroke syndrome, such as acute ischemic stroke (AIS), intracranial hemorrhage (ICH), and transient ischemic attack (TIA), and some of the potential challenging differential diagnoses in the acute emergency setting. It also reviews our institutional technical and clinical experience in MR imaging of patients with acute stroke and provides some clinical examples.
Role of magnetic resonance imaging in stroke imaging
The primary goals of neuroimaging are to determine the presence of infarction and to distinguish between hemorrhagic and ischemic stroke. The secondary goals of stroke imaging, largely applied to ischemic strokes, are to identify the location and extent of intravascular clot as well as the presence and extent of penumbra (hypoperfused tissue at risk for infarction). To be effective, comprehensive stroke protocols should be able to address the aforementioned primary and secondary goals in a timely manner.
Although CT is the most commonly used modality for stroke imaging, partly because of its wide availability and faster acquisition time, some comprehensive stroke centers choose MR imaging rather than CT for 2 major reasons. First, higher sensitivity and specificity of MR imaging for delineation of hyperacute ischemia. Diffusion-weighted imaging (DWI) provides the most specific way to image acute infarction and perfusion imaging can help in delineation of ischemic penumbra. The advent of MR imaging has redefined stroke syndromes such as acute ischemic infarction and TIA from an all-or-none process to a dynamic and evolving process, providing meaningful physiologic and functional information. Second, the absence of radiation. A comprehensive CT stroke protocol delivers a mean effective dose of 16.4 mSv, which is approximately 6 times the dose of an unenhanced CT head. This difference is particularly important for patients who need repeat examinations following treatment or have a change in their neurologic examination, in whom the repeated CT scans can be prohibitive because of the accumulated radiation dose.
How I do it?
Image Acquisition (Technical Aspects)
At our institution, if there is no contraindication, MR is the default imaging modality for patients presenting with suspicion of AIS. After activation of the stroke code and the patient’s arrival at the emergency department, an MR safety questionnaire is administered and MR-compatible electrocardiogram leads are placed as the patient is being evaluated by the neurology team. The patient is then placed on an MR-compatible table and wheeled to the MR magnet for imaging.
We have 2 stroke MR protocols in place: (1) a fast stroke protocol that takes approximately 6 minutes to acquire, and (2) a routine stroke protocol that takes about 20 to 25 minutes. Although many factors contribute to the decision of which protocol to perform, the fast MR protocol is mainly used for patients who are considered strong candidates for an interventional procedure, such as mechanical embolectomy, to minimize the imaging time without delaying treatment. The routine stroke protocol encompasses other imaging components that allow for further detailed evaluation of the brain, taking into account other differential diagnoses and stroke mimics.
Improvements in MR imaging hardware technology, including the introduction of multicoil technology for better signal reception and higher magnetic fields (≥3 T), which afford higher signal/noise ratio, have increased the efficiency with which fast imaging tools can be applied. In addition, fast sequence design such as echo planar imaging (EPI) and rapid imaging tools such as parallel acquisition algorithms have resulted in significant improvements in the efficiency of MR imaging in terms of both spatial and temporal resolution. Taking advantage of these combinations, we have designed and are effectively using a fast MR imaging protocol with total acquisition time of approximately 6 minutes, a 4-fold reduction in scan time compared with conventional MR stroke imaging. For comprehensive stroke centers that choose MR imaging as their imaging modality, the described protocol allows a comparable acquisition time and efficiency to that of multimodal CT protocols, and takes advantage of the superior tissue resolution, the higher sensitivity, and the higher specificity for delineation of infarction afforded by MR imaging.
Imaging components
Comprehensive MR stroke protocols used routinely in major stroke centers have 3 essential components: (1) parenchymal imaging, which identifies the presence and size of an irreversible infarcted core, determines presence of hemorrhage, and helps to age the ischemic event; (2) MR angiogram to determine the location of arterial occlusion and presence of an intravascular thrombus that can be treated with thrombolysis or thrombectomy; (3) perfusion imaging to determine the presence of hypoperfused tissue at risk for subsequent infarction if adequate perfusion is not restored.
Each of these components and their potential clinical applications are reviewed here.
Parenchymal Imaging
Parenchymal imaging usually encompasses 3 components:
Diffusion-weighted imaging
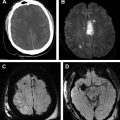
Diffusion-weighted imaging (DWI) can detect ischemic tissue within minutes of ictus and has emerged as the most sensitive and specific imaging technique for acute ischemia ( Fig. 1 ), far beyond nonenhanced CT or any other type of MR imaging sequences. In addition, the pattern of the DWI abnormalities provides insight into the underlying cause and stroke subtype. For example, visualization of multiple small bright lesions on DWI sequences within different vascular territories may indicate an embolic stroke mechanism.
Fluid-attenuated inversion recovery
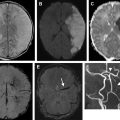
Fluid-attenuated inversion recovery (FLAIR) helps to determine the age of the infarction, permits detection of subtle cerebral subarachnoid hemorrhage, and can add diagnostic value to gradient-echo (GRE) images for detecting intra-arterial clot. The most important use of FLAIR imaging in the setting of acute stroke is to identify acute ischemic infarcts that lie within the thrombolytic time window in patients with symptoms first noted on awakening (wake-up stroke), or patients with unwitnessed onset who are unable to provide an accurate history. As a rule, lesion visibility on FLAIR increases as time passes from the stroke onset and up to 93% of acute stroke lesions can have positive FLAIR findings at greater than 6 hours. FLAIR sequences may also show hyperintense vessels following a proximal occlusion even in the absence of parenchymal signal changes. These findings may indicate slow flow in the collateral circulation. In our 6-minute stroke protocol we have replaced conventional FLAIR with EPI-FLAIR. We have shown that EPI-FLAIR has similar diagnostic performance and quantitative and qualitative results to conventional FLAIR ( Fig. 2 ), but only requires one-third of the acquisition time. Because of the shorter acquisition time, EPI-FLAIR may provide better image quality with less motion artifact, particularly in uncooperative patients ( Fig. 3 ).
Gradient-echo imaging
GRE imaging is used to detect intracranial hemorrhage and intraluminal thrombus formation ( Fig. 4 ). Although CT is the standard method used to rule out intracranial hemorrhage, GRE has been shown to be at least as accurate as CT for the detection of acute intraparenchymal hemorrhage. Both FLAIR and GRE images have been used to detect intra-arterial clot with variable sensitivity and specificity. Again, in our 6-minute stroke protocol, EPI-GRE has replaced conventional GRE with similar diagnostic performance but only a fraction of the acquisition time (see Fig. 2 ).