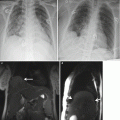
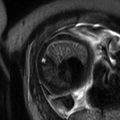
Fig. 17.1
This 37-year-old woman G3P2 presented at 24 weeks’ gestation with increasing shortness of breath. Coronal gated spin echo (a) and (b) axial cine gated gradient echo images demonstrated a 5 cm right atrial pedunculated mass (*). She underwent operative delivery at 28 weeks followed by removal of a right atrial myxoma
In the CHIRP study (Cardiovascular magnetic resonance in pregnancy) by Ducas and colleagues [25], prospective comparison between MRI and transthoracic echocardiography demonstrated that there was good correlation between data on left ventricular mass, stroke volume, and cardiac output. Interestingly, transthoracic echocardiography was found to consistently underestimate the values. This study provides us with reference cardiac indexes for normal pregnancy for future comparison to various pathological conditions.
17.3 Basics of Cardiac MRI in the Pregnant Patient
Although a variety of both traditional and experimental MRI protocols can be used to assess the cardiovascular system, a few key protocols are essentially the workhorses of cardiovascular MRI. Basic anatomical assessment is traditionally carried out with black blood and bright blood imaging. In black blood imaging, a spin echo technique, the cardiac tissues demonstrate high signal intensity. The blood possesses low signal intensity, therefore appearing black. In bright blood imaging, a gradient echo technique, the cardiac tissues possess low signal intensity and the blood pool is bright, possessing high signal intensity. Not only is the bright blood technique good for anatomical assessment, it also allows evaluation of left ventricular and valve function as well as assessment of any intracardiac shunts. Similar to the bright blood technique, steady-state free precession is sometimes used to generate cine images with excellent temporal and spatial resolution. To complete functional assessment of the valves and any intracardiac shunts, flow velocity encoding technique or phase contrast is often used.
Cardiac and respiratory gating may be necessary to ensure good diagnostic quality of the images. Traditionally, cardiac gating is used throughout the imaging acquisition. Presence of atrial fibrillation or excessive irregular beats can interfere with cardiac gating and degrade image quality. Presence of respiratory motion can also degrade image quality. Either breath-hold technique or respiratory gating should be utilized depending on the length of time needed for image acquisition of each sequence. Alternatively, ultra-fast “real-time” cardiac MR may be performed without cardiac or respiratory triggering [60].
Particular attention should be paid to patient positioning. The gravid uterus may cause venous compression and therefore affect baseline hemodynamic function. A study performed by Rossi and colleagues [69] concluded that patient position affects venous return, stroke volume, and cardiac output. It is suggested that starting at the 20th week of gestation, patients should be positioned in the left lateral position, rather than the supine position. This is of utmost importance in patients that may receive serial exams throughout pregnancy. Positioning should remain in the left lateral position to assure changes in function are not confounded by the supine position.
The CHIRP study [25] detailed MRI findings in normal healthy pregnant patients. It is important to understand the expected physiological changes that occur during pregnancy and the associated finding on MRI. The authors demonstrated an increase in left ventricular end-diastolic volume and left ventricular end-diastolic diameter in the third trimester. There was also a significant increase in left ventricular mass. Increase in both heart rate and stroke volume resulted in calculated increase in cardiac output in the third trimester.
The CHIRP study also demonstrated significant increase in right ventricular end-diastolic diameter, right ventricular mass, and right ventricular volume. Additionally there was biatrial enlargement in the third trimester. Interestingly, there was no change in the size or geometry of the aortic root or ascending aorta during pregnancy.
17.4 Evaluation of Congenital Cardiovascular Diseases
17.4.1 ASD
Atrial septal defects (ASDs) are a fairly common congenital heart anomaly and have been found to make up approximately 13 % of congenital heart disease worldwide [76]. Abnormalities occurring during embryonic formation of the heart during organogenesis can result in a variety of different defects. The two most common types of ASD are primum ASD and secundum ASD. Primum ASD occurs with a defect in the most apical section of the intra-atrial septum. In this defect, there is an abnormality in the development of the endocardial cushions. This type of ASD is often associated with malformation of the atrioventricular valves as well as ventricular septal defects. The secundum type of ASD results from halted formation of the septum secundum. Alternatively, this defect also may result from over-absorption of the septum primum. Secundum ASDs are far more common than primum types and are seen twice as frequently in women [43]. ASDs can also involve the sinus venosus and the coronary sinus. Sinus venosus ASDs are quite rare, demonstrating connection between the superior or inferior vena cava and the right pulmonary veins. Ganigara and colleagues [34] point out that diagnosis can be particularly challenging, and MRI is an essential diagnostic tool. Involvement with the coronary sinus is considered the most rare type. In this form, an unroofed coronary sinus connects directly with the left atrium.
The clinical presentation of patients with ASDs can be widely varied depending on the size of the defect and the resultant potential left-to-right shunting of blood. Although some patients may be asymptomatic for quite some time, physiological situations, such as pregnancy, which increase demand on the cardiovascular system, may result in symptomatic presentation. Common symptoms include atrial arrhythmias or general fatigue, as well as dyspnea. On physical exam, patients may demonstrate parasternal right ventricular lift. Classically, on auscultation, a fixed split of the S2 heart sound can be heard. Although a systolic ejection murmur is often present, this can be easily overlooked as it may mimic the physiological murmur of pregnancy. During pregnancy, large ASDs may result in congestive heart failure, or the patient may also develop atrial arrhythmias, prompting a diagnostic workup. Given the hypercoagulable state of pregnancy, the development of peripheral deep vein thrombosis is of particular concern given the theoretical risk of paradoxical embolus.
Depending on the acuity of the clinical presentation, women may receive initial medical diagnostic workups that include EKGs and, in isolated circumstances, a chest radiograph. EKG may show a partial right bundle branch block. Depending on the size of the defect, initial radiograph may demonstrate enlargement of the right heart and signs of pulmonary vascular congestion. Echocardiography is the diagnostic test of choice for evaluation of the location and extent of the defect. However, in more complicated cases cardiac MRI is useful. In fact, more recently MRI is becoming an essential integral part of the complete diagnostic workup [67]. Of particular benefit, Beerbaum and colleagues [9] demonstrated that cardiac MRI could be used to quantify blood flow using the phase-contrast cine technique. Cardiac MRI can provide detailed assessment of defect size and location and also aid in detection of other associated conditions such as anomalous pulmonary venous return. Furthermore, MRI can be particularly useful in diagnosing the more rare types of ASD such as coronary sinus ASD [15]. In directly comparing cardiac MRI to transesophageal echocardiography, Piaw and colleagues [64] demonstrated cardiac MRI cine balanced fast field echo and phase-contrast technique to correlate well with measurements obtained via echocardiography and provide the added benefit of additional anatomical information.
Alpendurada and colleagues [3] recommend beginning the initial MRI evaluation with anatomical images in the transaxial, coronal, and sagittal planes using a sequence such as single-shot turbo spin echo and single-shot balanced steady-state free precession. Cine images are also recommended for adequate anatomical evaluation. For ASDs in particular, a short-axis stack in the atrioventricular plane may be particularly critical for accurate evaluation. Finally, flow measurements can be used to assess the dynamic impact of the ASD on cardiac function. Similarly, Gulati and colleagues [42], in describing how their institution evaluates ASDs, suggest axial black-blood-prepared fast spin echo images. This is followed by bright blood steady-state free precession cine sequences. Finally velocity-encoded cine sequences are used to assess flow.
In pregnant patients requiring acute invasive intervention, such as percutaneous transcatheter ASD closure, MRI has proven to be a valuable tool in assessing the impact of intervention. For example, Burgstahler and colleagues [13] demonstrated the use of MRI to accurately evaluate cardiac function post ASD closure. For more detailed and accurate assessment, Chen and colleagues [16] suggest a technique in which, following anatomical imaging acquisition, a cine gradient echo-planar sequence is used with a labeling pre-pulse to tag across the basal myocardium. Right ventricular tag displacement can then be measured and tracked to standardize measurement of right ventricular function.
17.4.2 VSD
Ventricular septal defects (VSDs) while considered the most common congenital heart defect are more rare in the adult population due to the common occurrence of spontaneous closure. As classified by Morello [47] there are four main types of VSD, divided according to anatomical location. Type 1 demonstrates infundibular septum defects. In this type there is a septal deficiency between the crista supraventricularis and the aortic and pulmonary valves. Aortic regurgitation is common. Type 2 defects are present in the membranous septum. This is considered the most common type of VSD. If the defect extends into the muscular septum, this may be classified as a perimembranous VSD. Type 3 VSD demonstrates a defect in the inlet septum. The defect is therefore located below the level of the mitral and tricuspid valves. Type 4 VSD demonstrates defects in the muscular septum. This type is not located in close proximity to the cardiac valves. An atrioventricular VSD, or Gerbode defect, is characterized by a defect in the septum separating the right atrium from the left ventricle. This type is exceptionally rare.
Severity of clinical symptoms depends on the size and location of the defect and the resultant severity of left-to-right shunting of blood. Although most women are asymptomatic throughout pregnancy, larger defects may potentially result in symptoms associated with left ventricular overload including fatigue and dyspnea. Development of pulmonary hypertension or aortic regurgitation is also a potential result of larger VSDs. In a study by Karamlou and colleagues [50], women with VSDs in particular, who are at increased risk of mortality and peripartum complications, often do not receive diagnostic work and care at academic centers. Proper diagnostic workup and treatment are critical given the known increased risk of preeclampsia, premature labor, and small for gestational age births in women with unrepaired VSDs [80].
Diagnostic workup often begins with an EKG demonstrating left ventricular hypertrophy. Chest radiography (if acquired) often shows nonspecific signs such as increased pulmonary vascular congestion and left heart enlargement. Echocardiography is often the diagnostic imaging test of choice to visualize and classify the defect. However, MRI may be necessary for more thorough evaluation and determining need for and timing of potential interventions. MRI allows for a more detailed anatomical assessment of the pulmonary vasculature and allows for quantification of left-to-right shunting using phase-contrast cine technique [24]. Specific techniques and recommended sequences are similar to that detailed in evaluation of the ASD.
17.4.3 PDA
A patent ductus arteriosus (PDA) can occur when the ductus arteriosus, or fetal connection between the aorta and the main pulmonary artery, fails to undergo natural postnatal closure. Although most PDAs are detected and treated early in childhood, a few may persist undetected into adulthood. Depending on the size of the PDA and the resultant left-to-right shunt, there is a potential risk for the development of elevated pulmonary vascular resistance. If the defect is significantly large, patients may present in preeclampsia and heart failure due to the increased cardiovascular demands of pregnancy [2].
Initial diagnostic workup often includes a chest radiograph demonstrating an enlarged left ventricle and pulmonary artery as well as increased pulmonary vascular congestion. If the PDA is small, the radiograph may appear within normal limits. Occasionally calcification of the ductus may be seen on lateral radiograph. Classically, the echocardiography workup shows continuous flow within the pulmonary artery [28]. MRI is useful for more complicated cases and for surgical planning. MRI allows accurate description of the size of the PDA and degree of calcifications [4].
Because many PDAs are identified and treated in childhood, the treatment has the potential to impact the patient throughout their adult life. Many of the treatment devices and coils are not MRI-compatible and therefore limit diagnostic evaluation of the patient during the pregnant and peripartum periods. Grifka and colleagues [40] demonstrate use of non-ferromagnetic embolization coils, used for transcatheter PDA closure, therefore enabling future successful evaluation with cardiac MRI.
17.4.4 Aortic Stenosis
Valvular aortic stenosis (AS) has a wide variety of potential underlying causes. Of note, stenosis can occur at the sub- and supravalvular levels in addition to the valvular level. Congenitally, AS may result from an abnormal unicuspid or bicuspid valve which develops calcifications. Alternatively, calcifications can develop on a trileaflet valve, resulting in stenosis. Rheumatic heart valve disease may also result in AS, discussed later in this chapter. AS is monitored and classified based on the antegrade velocity of blood across an abnormal valve, resulting in varying degrees of obstruction to left ventricular ejection.
AS can be asymptomatic until the outflow obstruction becomes hemodynamically significant. Symptoms are relatively nonspecific and include shortness of breath, presyncope/syncope, or angina. Ideally, AS is recognized and treated prior to pregnancy, as mortality and morbidity are increased in patients with AS during pregnancy and the peripartum period. Furthermore, women with severe AS who are symptomatic during pregnancy are at increased risk of requiring cardiac interventions postpartum [75]. The 2008 update of the 2006 ACC/AHA guidelines notes severe AS as a condition associated with high maternal risk during pregnancy. Furthermore, the guidelines suggest counseling to delay conception until after treatment.
Diagnostic workup often includes EKG and chest radiographs. Chest radiographs may be normal. Alternatively, one might see an enlarged left ventricle or post-stenotic aortic dilatation. Diagnosis and evaluation are routinely made by echocardiography, which allows for detailed analysis of the aortic valve. Although echocardiography is the gold standard for assessing the aortic valve, cardiac MRI has proven to be useful in assessing associated features. Cardiac MRI has been used for assessment of left ventricular volume and function. Furthermore MRI allows for more detailed assessment of the myocardium.
17.4.5 Mitral Valve Prolapse
Prolapse of the mitral valve can result in varying degrees of mitral regurgitation. Prolapse may be congenital or acquired in nature. Prolapse is technically defined as one or both mitral valve leaflets projecting into the left atrium during systole. This may be due to abnormalities in or disruption of the valve leaflets, the chordae, or the papillary muscles. A component of myxomatous degeneration of the valve leaflets may also be present. Mitral valve prolapse is present frequently, and the associated mortality and morbidity with the pregnant and peripartum periods are relatively low. However, one must consider the possibility of the cardiac demands of pregnancy precipitating an arrhythmia. Additionally, there is a risk of infective endocarditis, for which prophylactic antibiotics may be clinically indicated.
Although echocardiography is the most appropriate first step in assessment of the mitral valve and evaluation of degree of regurgitation associated with valve prolapse, MRI is emerging as a valuable tool to aid in diagnosis. Not only does MRI provide accurate assessment of the mitral valve function, it also often elucidates the underlying cause for functional abnormality. Cardiac MRI provides excellent insight into the geometry of the mitral annulus. As detailed by Morello and Gelfand [59], the degree of mitral valve prolapse is often best assessed by oblique coronal steady-state free precession imaging of the left ventricular outflow tract or alternatively in the four-chamber view. MRI can further be used to quantify severity of regurgitation. This can be assessed with volumetric methods or flow velocity mapping. The precise volume of mitral regurgitation can even be obtained through subtraction of the aortic flow from left ventricular inflow [59]. Cardiac MRI can also be used to assess the long-term effects of mitral regurgitation. Left ventricular hypertrophy and other changes in right ventricular geometry can be accurately detailed.
17.4.6 Coarctation of the Aorta
Coarctation of the aorta is generally defined as descending aorta narrowing, commonly at the insertion sites of the ductus arteriosus distal to the left subclavian artery. This anatomical variation, a congenital anomaly, results in left ventricular pressure overload. Of note, coarctation is usually present with other cardiac anomalies such as abnormal valves, ASD, or VSD.
If the anomaly is not diagnosed until adulthood, the most common clinical presentation is hypertension. In severe cases, sequel of hypertension such as stroke or aortic dissection can also be seen. On physical exam, there is a difference in systolic blood pressure between the upper and lower extremities. The upper extremities display hypertension, while the lower extremities are often hypotensive.
ECG is most often normal. Chest radiograph demonstrates typical findings such as rib notching and contour indentation of the aorta at the coarctation site, producing the classic “3” sign. Echocardiography is often the first advanced imaging modality of choice. However, the [12] ACC/AHA adult congenital heart disease guidelines specify that every adult with known coarctation, regardless of repair, is required to have at least one cardiac MRI (or CT) for a complete diagnostic workup. MRI allows for detailed anatomical analysis of the location and extent of the coarctation. Furthermore, MRI allows for mapping of collateral vessels. When the diagnosis is known prior to conception, MRI has been shown to allow for prediction of increased risk for hypertensive events. Jimenez-Juan and colleagues [48] demonstrated that smaller aortic dimensions correlated to increased risk of hypertensive events during pregnancy in women with coarctation of the aorta. MRI has also been proven as an accurate tool to predict catheterization gradient across a coarctation [61] and therefore an excellent alternative to angiography during pregnancy.
17.4.7 Pulmonary Stenosis
Pulmonary stenosis can occur at the valvular, subvalvular, or supravalvular level. Usually, a trileaflet valve with fibrous thickening is identified in a patient with congenital pulmonic stenosis. The leaflets are often described as having a characteristic “fish-mouth” appearance. PS is often associated with right ventricular hypertrophy. Rarely, stenosis can be seen in a bicuspid valve.
This anomaly is often missed in childhood and may present later in adulthood with increased cardiovascular demand such as pregnancy. Patients may present with fatigue, presyncope/syncope, or angina. On auscultation, one might detect splitting of the second heart sound or a right-sided fourth heart sound. Echocardiography is considered the gold standard for assessment of the pulmonic valve. In general, PS does not present with significantly increased mortality or morbidity in the peripartum period.
Although echocardiography is traditionally the gold standard for diagnostic workup, MRI can be of use for more complex cases or for planning intervention. Dedicated sequences through the pulmonary valve can be obtained using steady-state free precession for an accurate analysis of valve function. Flow across the valve is analyzed with velocity-encoded phase-contrast images. Furthermore, pulmonary vasculature can also be assessed with a 3D spoiled gradient echo angiographic sequence [66]. MRI cine images demonstrate thickened valve leaflets that take on a dome-shaped appearance due to restricted motion. Furthermore, cine images often show a dark jet across the pulmonic valve due to dephasing caused by high velocity [66].
17.4.8 Tetralogy of Fallot
Tetralogy of Fallot, first described in 1889 by Étienne-Louis Arthur Fallot, is one of the most common congenital cyanotic heart conditions. Although detection and subsequent surgical repair are becoming increasingly more common shortly after birth, rarely women do present during pregnancy or the peripartum period with an unrepaired defect. Tetralogy of Fallot consists of four key anatomical abnormalities. First, patients demonstrate pulmonary stenosis. The point of maximal stenosis can vary but is most commonly in the infundibular position. Second, there is a presence of a usually large ventricular septal defect (VSD). Of note, there may be multiple VSDs present. The VSD is typically present in the perimembranous region and may extend into the muscular septum. Third, there is deviation of the aorta rightward such that the aorta overrides the VSD, receiving blood flow from both ventricles. Fourth, pulmonary stenosis results in right ventricular hypertrophy. Postoperative evaluation will be discussed later in this chapter.
Given the altered hemodynamic flow, patients are acutely sensitive to changes in systemic vascular resistance. Changes that occur throughout pregnancy and particularly in labor and delivery create a potential situation of acute cyanosis that carries a high risk of mortality and morbidity for both the mother and the fetus.
Workup generally begins with a chest radiography demonstrating a “boot-shaped” cardiac silhouette. Echocardiography is typically the primary method for diagnosis and evaluation. Partington and Valente [62] describe in detail how to approach evaluation with cardiac MRI. It is critical to visualize and evaluate the entirety of the right ventricular outflow tract (RVOT). There is a spectrum of varying severities of obstruction that is seen in tetralogy of Fallot. The RVOT should be visualized in long-axis as well as two-chamber cine views, thus allowing accurate assessment of the hemodynamic flow through the tricuspid valve. One should look carefully for RVOT aneurysms as well as valve abnormalities. If needed in the case of distal pulmonary stenosis, additional sequences such as MRA can be added for further assessment of the pulmonary artery. Particularly in the peripartum period, detailed and accurate assessment of right ventricular function is needed. Potential dysfunction of the left ventricle should also be evaluated. Often found concurrently with RVOT, pulmonary regurgitation can be easily identified and assessed with cardiac MRI. A plethora of variations and anomalies can be found in tetralogy of Fallot, and cardiac MRI is excellent for providing detailed anatomical assessment. One can assess for variations in coronary anatomy, presence of a right-sided aortic arch, and anomalies in the pulmonary arteries. Cardiac MRI can provide critical diagnostic details, providing anatomical information about the entire heart, valves, and many associated vascular anomalies.
17.4.9 Ebstein Anomaly
Ebstein anomaly results from congenital malformation of the tricuspid valve and right ventricle. Normally, the valve consists of three leaflets: the anterior, posterior, and septal. In Ebstein anomaly, the leaflets extend below the anatomical annulus and form varying types of attachment to the ventricle. The leaflets themselves often display abnormal morphology with a wide spectrum of dysplasia and dysfunction. Several classification systems have been proposed to describe the wide range of pathology seen in this condition encompassing both anatomical and functional descriptions (see [35] for a review). Most recently, and perhaps most relevant to radiographic descriptions, a classification was proposed by Derani and Danielson [23] which incorporates both echocardiographic appearance and findings at the time of surgical evaluation, grading the defect I, II, III, or IV.
Although this congenital cyanotic heart anomaly is often recognized in infancy or childhood, it may have rare presentation in the adult population. In adults, Ebstein anomaly is most likely to present with arrhythmias. Certainly the increased cardiovascular demands of pregnancy can potentially precipitate symptoms such as arrhythmia.
If performed, initial chest radiography will often demonstrate features consistent with right atrial enlargement, and EKG will demonstrate congruent findings and frequently demonstrates right bundle branch block. More thorough evaluation is generally performed with echocardiography. However, MRI can be a useful tool for evaluation of more complex cases. MRI allows for further detailing of the tricuspid valve, detailing the severity of tricuspid regurgitation and providing functional data on the ventricles as well as ventricular volume. If present, as in some cases, the extent of right-to-left shunt can also be assessed. In Ebstein anomaly, the right ventricle is divided into two portions. The atrialized portion lies above the attachment of the abnormal leaflets, and the remaining functional right ventricle lies below the leaflet attachments. Cardiac MRI allows for full analysis of the atrialized portion, functional portion, and total right ventricle through the use of cine four-chamber views and axial and short-axis views [35].
17.4.10 Tricuspid Atresia
In tricuspid atresia there is either congenital absence or agenesis of the tricuspid valve. This results in lack of communication between the right atrium and right ventricle. There are varying types of atresia with classification based on the anatomical findings. There is, for example, muscular atresia, membranous atresia, and valvular atresia. Valvular anomalies are always associated with an atrial septal defect that allows communication of blood from the right to the left ventricle. There is also, due to alteration in hemodynamic flow, right ventricular hypertrophy. Quite often, there is a VSD as well. Other potential associations include obstruction of the pulmonary outflow tract and a hypoplastic pulmonary artery. The great arteries may be in normal anatomical position or transposed. Tricuspid atresia is generally classified into three types, based on anatomical findings.
Rarely, tricuspid atresia goes undetected into adulthood; however there have been several case reports of adults successfully delivering babies with uncorrected tricuspid atresia [10]. Cardiac MRI can be helpful in distinguishing tricuspid atresia from Ebstein anomaly. Initial imaging with chest radiography demonstrates nonspecific findings depending on pulmonary blood flow. Sometimes, findings consistent with right atrial enlargement are present. Patients are typically evaluated with echocardiography. However, MRI is becoming an increasingly more essential piece of the diagnostic workup, especially in pre- and postsurgical evaluation. MRI can provide essential information about ventricular volume and shunt dynamics, as well as provide detailed anatomical information about the valves. MRI is particularly useful in differentiating the different types (I-IV) of tricuspid atresia based on the signal intensities of the atrioventricular sulcus [32].
17.4.11 Transposition of the Great Vessels
Transposition of the great vessels is most commonly seen in the dextro variant, in which the pulmonary artery arises from the left ventricle and the aorta from the right ventricle.
Because transposition of the great vessels is nearly always detected and surgically corrected in infancy, the relevance in its native state during pregnancy and the peripartum period is small.
17.4.12 Truncus Arteriosus
In truncus arteriosus, a single large trunk arises from both ventricles and gives rise to both the aorta and the pulmonary arteries. In this cyanotic congenital lesion, there is a single semilunar valve, which can demonstrate a wide variety of morphologies. This anomaly is typically associated with a ventricular septal defect. Variant anomalies of the aorta, such as a right-sided aortic arch, are also commonly seen with truncus arteriosus. It is exceedingly rare for a patient to make it to childbearing age with this congenital lesion without some type of surgical correction.
If patients do survive into adulthood without surgery, they typically display clinical symptoms consistent with Eisenmenger syndrome (discussed later), which carries a high morbidity and mortality when associated with pregnancy. Cardiac MRI can be a critical tool for providing anatomical and functional information. As explained by Fogel and Crawford [33], detailed analysis using steady-state free precession, cine imaging, and velocity mapping allows for evaluation of the exact type of common trunk as well as branches and collateral vessels. Functional assessment of the semilunar valve is also achieved. Determining aortic arch hypoplasia or interruption is particularly important for determining where the lesion falls in the different classification schemes.
17.4.13 Single Ventricle
Patients with a single ventricle have a single large ventricular chamber that received blood from both the right and left atria. This rare condition is often associated with transposition of the great vessels and with pulmonary stenosis. Although there are scattered successful pregnancies reported, this lesion carries with it high peripartum morbidity and mortality for both the mother and the fetus. Patients are at greatest risk for thromboembolic disease and congestive heart failure. Use of MRI can profile critical information about the ventricular volume and function prior to and following any surgical intervention.
17.4.14 Eisenmenger Syndrome
Eisenmenger syndrome encompasses a spectrum of congenital lesions, all of which result in severe pulmonary hypertension and pulmonary vascular obstructive disease. Lesions producing this effect, including ASDs, VSDs, and PDAs, allow mixing of systemic and pulmonary circulation and therefore cyanosis. Pulmonary vascular occlusive disease results in shunt reversal or bidirectional shunting. In this rare condition there is a high risk of maternal mortality and morbidity during the peripartum period [53]. Women with this condition are recommended to avoid conception. As systemic vascular resistance decreases during pregnancy, there is an increase in the right-to-left shunting of blood, resulting in decreased arterial oxygen saturation. Ultimately, this creates heightened hypercoagulability and increases risk of thromboembolism. The sudden hemodynamic changes that occur during labor and delivery are poorly tolerated and particularly dangerous in this condition.
Detailed diagnostic evaluation of Eisenmenger syndrome is of utmost importance. Given the profound risk of both maternal and fetal mortality and morbidity with this condition, a diagnosis of Eisenmenger syndrome may lead some to consider termination of a pregnancy. Furthermore, accurate diagnosis during the prepartum period is important, as it will influence a patient’s decision to proceed with conception.
Initial chest radiography often demonstrates enlargement of the pulmonary arteries with peripheral pruning of the vasculature. On cardiac MRI, there is evidence of right ventricular hypertrophy and often paradoxical bulging of the septum toward the left ventricle. As the disease increases in severity, one may expect to see right ventricular enlargement and evidence of hypokinesis on cine imaging. Detailing the anatomical defect, allowing the shunt, and quantifying the degree of shunt can be done with MRI. Furthermore, the tricuspid and pulmonic valves can be evaluated for regurgitation. Full anatomical evaluation with MRI as well as other imaging modalities allows for accurate clinical decision-making and prognostication.
17.4.15 Marfan Syndrome
Marfan syndrome is an autosomal dominant connective tissue disorder. This disorder affects the entire body, with notable pathology often appreciated in the eyes, cardiovascular system, musculoskeletal system, pulmonary system, and skin. Specifically, within the cardiovascular system, there is often aneurysmal dilatation of the aortic root. Aortic valvular regurgitation may be present. Mitral valve prolapse may also be present with abnormal morphology of the mitral leaflets.
Due to the inherent abnormal dilatation of the aortic root in Marfan syndrome, peripartum patients are at particularly high risk of aortic dissection, aortic regurgitation, or even aortic rupture. Please see Fig. 17.2. Increased risk of dissection is multifactorial, likely due to changes in hemodynamics, shifts in hormonal balance, and the inherent deficit of aortic wall elastic fibers [39]. Chest radiography typically reveals dilatation of the thoracic aorta. In the pregnant patient, further anatomical evaluation with cardiac MRI is preferred over cardiac CT. Krishnam and colleagues [54] have found that use of unenhanced steady-state free precession magnetic resonance angiography can be used for accurate diagnosis and assessment of thoracic aortic disease without the need for gadolinium-based contrast agents. The 2010 [44] ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease point out that MRI may in fact be superior to echocardiography given its ability to fully characterize anatomical variants and collateral branches while also assessing valve pathology and providing data on left ventricular function. Functional data and measurements of aortic root dilatation allow for accurate decision-making regarding need for medical management or surgical repair. Should a complication such as aortic dissection occur during pregnancy or the peripartum period, cardiac MRI is a valuable tool for assessment and follow-up after medical or surgical management.

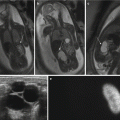
Fig. 17.2
A 27-year-old woman with Marfan syndrome with back pain and decreased right femoral pulse at 27 weeks’ gestation and history of mechanical aortic valve replacement. (a, b) Sagittal (a) and axial (b) gated steady-state free precession breath-hold MR images show sternal surgical artifact and mechanical aortic valve (oval, a), descending aortic dissection (asterisks indicate true lumen), fetus with placenta (p), and right iliac dissection extension (circle, b) (Reprinted with permission from the American Journal of Roentgenology Colletti et al. [19])
17.4.16 Absence of the Left Pericardium
Congenital absence of the pericardium is a rare but important diagnosis to accurately characterize. This congenital anomaly can either result in absence of the left pericardium or absence of the entire pericardium. While absence of the entire pericardium is typically asymptomatic and has a low mortality and morbidity rate in pregnancy, absence of the left pericardium can result in strangulation of the left atrial appendage. Patients can present with conspecific symptoms such as chest pain and presyncope/syncope. In the presence of other associated congenital abnormalities such as ASD, there is also a risk of emboli.
Chest radiography and echocardiography are not ideal for accurate evaluation of the pericardium. Therefore, in pregnancy MRI is the preferred diagnostic modality for evaluation of this congenital defect. In a case report by Yamano and colleagues [79], a patient presented with atypical chest pain. While radiography and echocardiography supported a diagnosis of complete absence of the pericardium, cardiac MRI allowed for more detailed evaluation and revealed absence of the left pericardium, prompting prophylactic surgical management.
17.5 Evaluation of Acquired Cardiovascular Diseases
17.5.1 Preeclampsia
Development of hypertension in pregnancy is a fairly common occurrence and prompts careful diagnostic workup and diligent clinical management to avoid potential maternal and fetal complications. Preeclampsia is diagnosed when a previously normotensive woman beyond 20 weeks’ gestation presents with new-onset hypertension and signs of end-organ dysfunction or proteinuria. Eclampsia is diagnosed with the development of seizures in a patient with preeclampsia. Although hypertension in pregnancy does not directly affect the myocardium in isolation, the change in hemodynamics creates potential functional changes that may become apparent on cardiac MRI evaluation. As detailed by Hamad and colleagues [65], the normal pregnant hemodynamic profile is characterized by increased cardiac output and decreased systemic vascular resistance. However, in preeclampsia there is decreased cardiac output and increased systemic vascular resistance [65]. This shift often leads to changes in left ventricular structure and function. Ultimately, the authors demonstrated impaired left ventricular function, increase in left ventricular wall thickness, and atrial enlargement in preeclamptic patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree