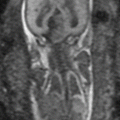
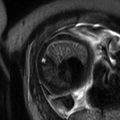
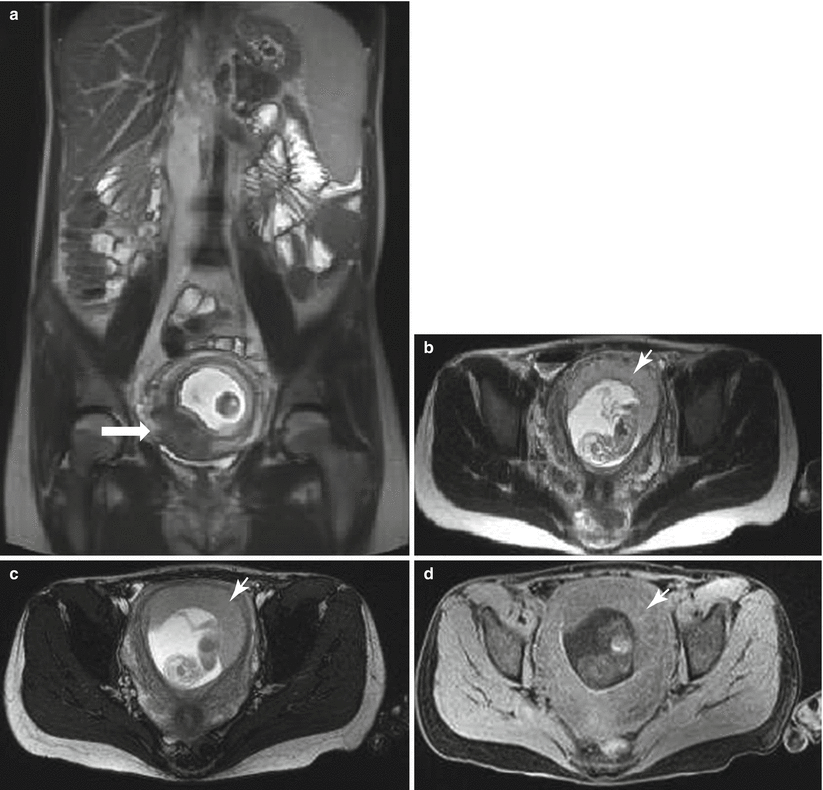
Fig. 13.1
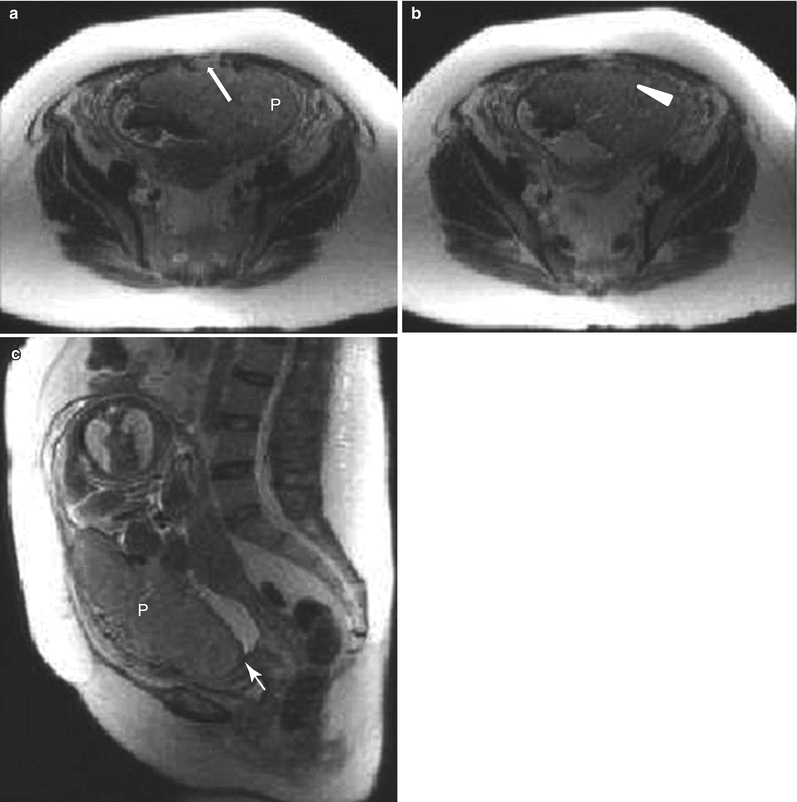
Uterine contraction in a 27-year-old at 6 weeks gestation referred for abdominopelvic MRI. Coronal (a) and axial (b) T2-weighted images demonstrate the early intrauterine pregnancy. No embryonic parts (fetal pole or yolk sac) are visualized, despite being present, which is normal during MR evaluation at this early gestational age. The coronal image, acquired earlier in the study, demonstrated a mass-like, low signal intensity area in the anterior myometrium (arrow) which resolved on subsequent images, consistent with a focal myometrial contraction. Focal myometrial contractions are a frequent finding on antenatal MRI and are distinguished from leiomyomas, which they resemble, by their transient nature
13.2 MRI of the Placenta
Given current practice paradigms, a request for dedicated MR evaluation of the placenta implies the presence of a sonographically detected abnormality (or potential abnormality). This increases pretest probability and necessarily introduces bias in placental evaluation on MRI. However, as with all imaging modalities, ultrasound remains an imperfect test subject to the skill and experience of the practitioner. Therefore, individuals interpreting MR imaging examinations of the placenta should review such studies objectively while availing themselves of all available imaging and clinical data.
T2-weighted multiplanar single-shot turbo/fast spin-echo sequences (e.g., rapid acquisition with relaxation enhancement [RARE] sequences such as half-Fourier acquisition single-shot turbo spin-echo [HASTE]) excel at revealing the internal structure of the placenta and underlying myometrium in addition to demonstrating fetal anatomy (Fig. 13.1). Sagittal imaging is imperative to evaluate the uterine serosa-bladder interface, but because the uterus is a curved structure and positioning of the placenta is variable, axial and coronal images are often helpful. We include non-breath-hold T1-weighted gradient echo images during initial evaluation of the entire gravid uterus at the beginning of the exam to ensure at least one T1-weighted sequence is available for review in case the patient cannot tolerate or continue the study. Later in imaging, higher-quality breath-hold three-dimensional T1-weighted spoiled gradient echo (GRE) images with fat saturation are used to further evaluate the placenta for hemorrhage, internal fat, and venous flow. We find that the entire placenta can be optimally evaluated within three short breath holds in the sagittal plane (Fig. 13.2). Care should be taken to minimize the number and duration of breath-hold acquisitions in gravid patients; therefore, we keep the length of breath holds under 16 s, especially in the third trimester. Balanced steady-state free precession sequences can help delineate placental lacunae, vessels, and masses (Fig. 13.3).
.
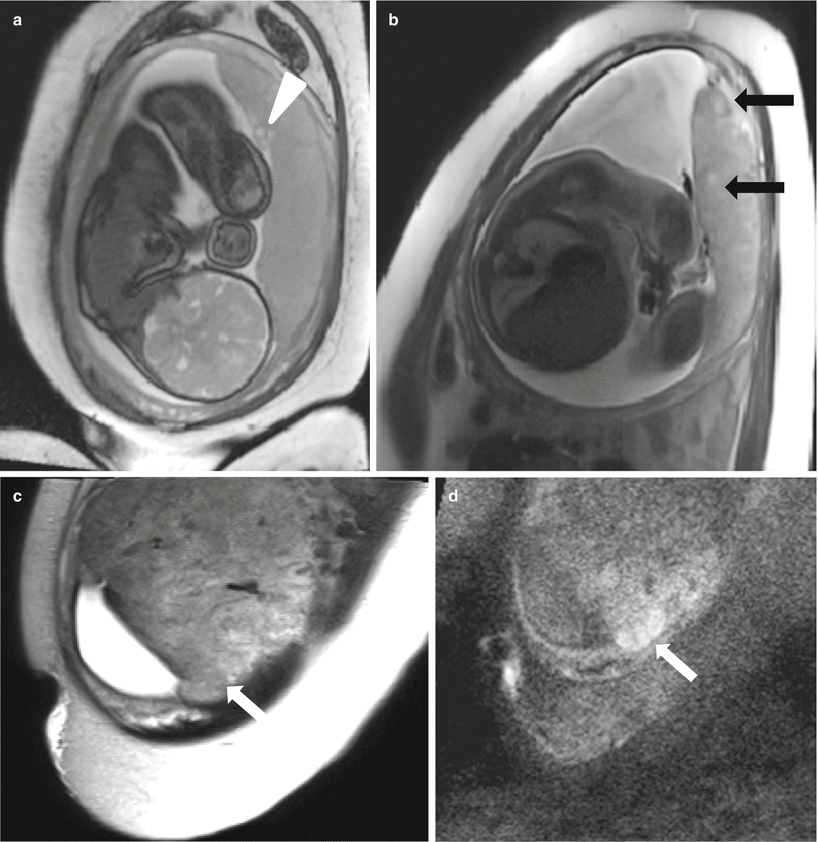
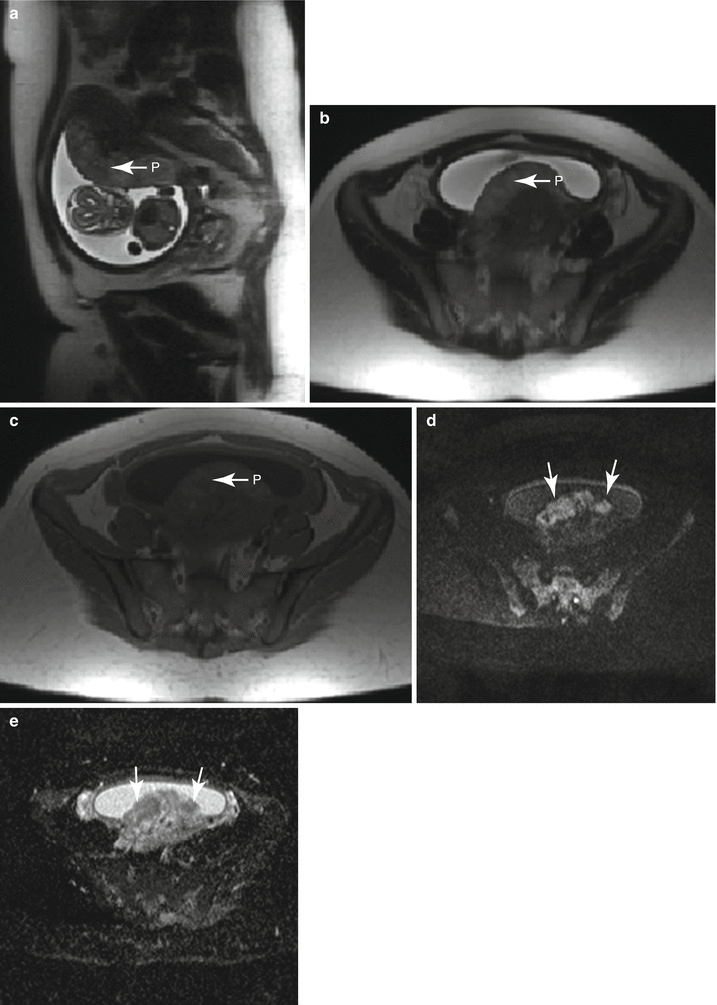

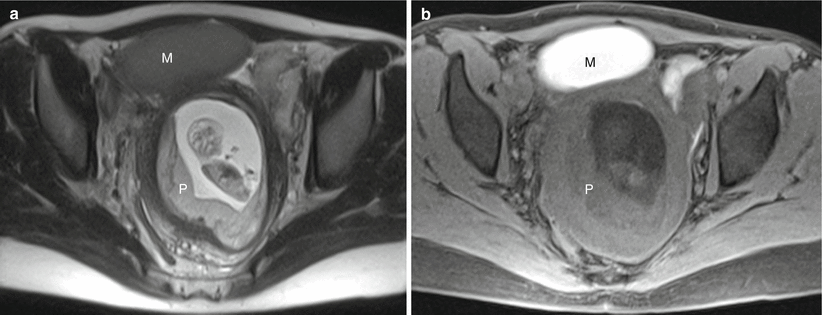
Fig. 13.2
Normal placenta in a 30-year-old G1 at 11 weeks gestation referred for MRI to evaluate bilateral adnexal masses. Axial RARE (a) and fat-suppressed GRE (b) sequences demonstrate a first trimester intrauterine pregnancy with a normal-appearing placenta (“P”). Note the large ovoid mass anterior to the uterus (“M”), determined to represent a large endometrioma

Fig. 13.3
Normal placenta in a 25-year-old at 13 weeks gestation referred for abdominopelvic MRI. Coronal (a) and axial (b) T2-weighted MR images demonstrate a late first trimester intrauterine pregnancy. A mass-like area in the anterior uterine wall (arrow) resolves on later imaging consistent with a focal myometrial contraction. Axial balanced steady-state free precession sequence (c) and fat suppressed T1-weighted GRE (d) images demonstrate the normal appearance of the placenta (small arrows) for this gestational age. Notice the anterior location, homogeneity, and smooth contour. The fetus is also readily visible at this point. Many placentas will be at least low lying in the first trimester, but distance between the placenta and the internal cervical os increases with gestational age in the majority of cases

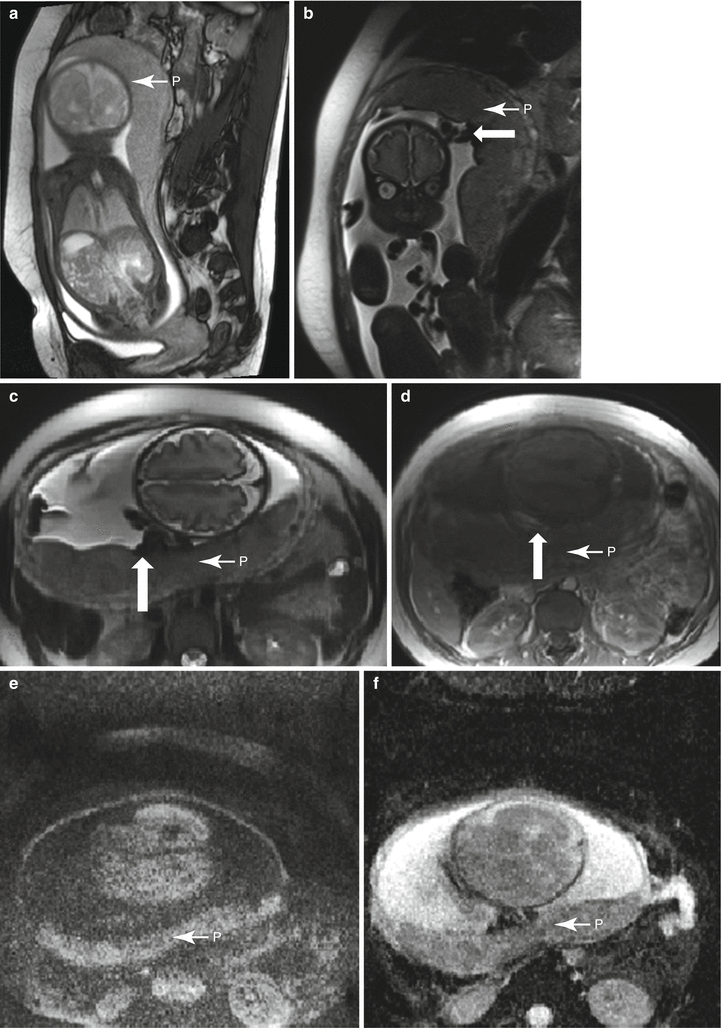
Fig. 13.4
Normal placenta in a 19 year-old at 32 weeks gestation referred for intracranial evaluation of a breech fetus. Sagittal balanced steady-state free precession sequence (a) demonstrates a normal-appearing fundal placenta (P). On RARE sagittal (b) and axial (c) images, the lobulation of the placenta is more apparent and the cord insertion (arrow) is well visualized. Axial T1-weighted image (d) demonstrates flow-related signal in the adjacent umbilical cord (arrow), not to be confused with preplacental hemorrhage. Axial DWI (e) and ADC (f) images demonstrate a normal pattern of signal intensity within the placenta, without focal restriction of diffusion or cotyledon formation

Fig. 13.5
Abnormal placental signal on diffusion-weighted images in a 27-year-old G5P1 at 21 weeks gestation referred for evaluation of the fetal posterior fossa; hypoplastic vermis with rotation (HVR) was identified (not shown). Sagittal (a) and axial (b–e) T2-weighted images demonstrate early heterogeneity in the signal of this second trimester posterior placenta (P). There were also lobular areas of heterogeneous T1 shortening identified (c) without focal hematoma. There were striking, globular areas of restricted diffusion (d) with correlating low ADC values (e) throughout the placenta, (arrows) an abnormal finding that has been related to intrauterine growth restriction (IUGR)

Fig. 13.6
Normal placenta in a 25-year-old at 36 weeks gestation with fetal ventriculomegaly and polyhydramnios. The fetus was found to have dysgenesis of the corpus callosum. Coronal balanced steady-state free precession sequence (a) demonstrates a slightly eccentric but still normal cord insertion (arrowhead) and developing lobulation of the maturing placenta. Axial T2-weighted image (b) shows early cotyledon development (black arrows). Although the cotyledon (arrows) are difficult to see on the coronal T2-weighted image (c), the diffusion-weighted image (d) in the same plane demonstrates increased signal in an organized, rounded shape typical of cotyledon formation in late third trimester (arrow)
We recommend that MR examinations performed for placental abnormalities be monitored by a physician who can prescribe additional sequences as needed, including fat-suppressed and opposed-phase imaging, if a fat-containing lesion is suspected, and time-of-flight imaging, if further evaluation of a vascular structure is indicated. Diffusion-weighted imaging (DWI), susceptibility-weighted imaging (SWI), and other functional MR evaluations have been attempted in the placenta but remain an area of active research for clinical applicability (Fig. 13.4). In a series by Bonel et al., restricted diffusion and reduced apparent diffusion coefficient (ADC) values were associated with placental dysfunction as can be seen in the setting of intrauterine growth restriction (IUGR) [14]. In the example of IUGR illustrated in Figure 13.5, the b-values used were 0 and 1000, and the ADC value in the region of most severe abnormality had a mean measurement of 137 s/mm2 (standard deviation 13 s/mm2) which would correlate with placental insufficiency using their criteria. Intravenous contrast is not routinely used in pregnancy and has not been needed for placental evaluation at our institution.
The normal placenta appears relatively homogeneous on T1- and T2-weighted images during the first and early second trimester (Fig. 13.1). With maturation, the placenta becomes more heterogeneous with undulating surface contours and internal rounded lobules representing early cotyledon formation. Care should be taken not to misinterpret the regular and organized cotyledon formation as indicative of disease (Fig. 13.6). The placental signal is typically homogeneously isointense to myometrium on T-2 weighted RARE sequences in early pregnancy and can become slightly hypointense to myometrium into the second trimester. It often remains homogeneous into the second trimester with early lobule formation occurring between weeks 24 and 31 [15]. With development of a lobular pattern, the placenta becomes less homogeneous in appearance as thin hypointense septa become apparent between the lobules on T2-weighted images. The underlying myometrial wall thins as the pregnancy advances, and this alone does not imply placental invasion [16]. Leiomyomas or myometrial contractions result in focal thickening, and often T2 shortening, of the myometrial wall. Myometrial contractions are distinguished from leiomyomas by the transient nature of contractions (Figs. 13.7 and 13.8). T1-weighted imaging provides insufficient soft tissue contrast within or between the placenta and myometrium to aid in diagnosis of abnormal placentation.
During MR evaluation of the placenta, hyperintense material may be identified within the endocervical canal on T1-weighted imaging. Often this can be related to normal cervical mucous, but hemorrhage can also have this appearance. Especially in the setting of previa, a small amount of T1 shortening within the endocervical canal is not unexpected but should be noted. Although the cervix normally shortens during the third trimester, it is especially important to note the apposed cervical length earlier in pregnancy if funneling or history of preterm labor is present.
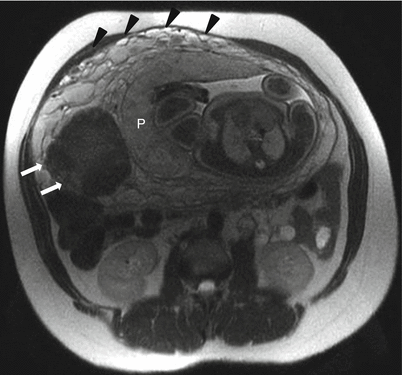
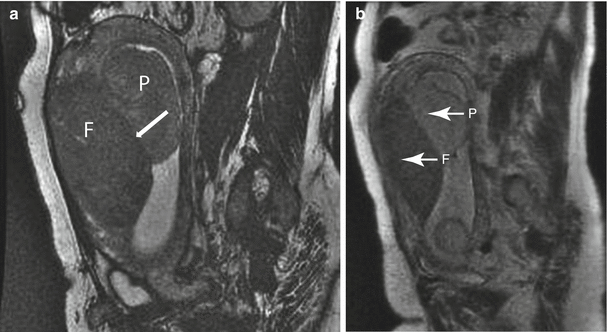
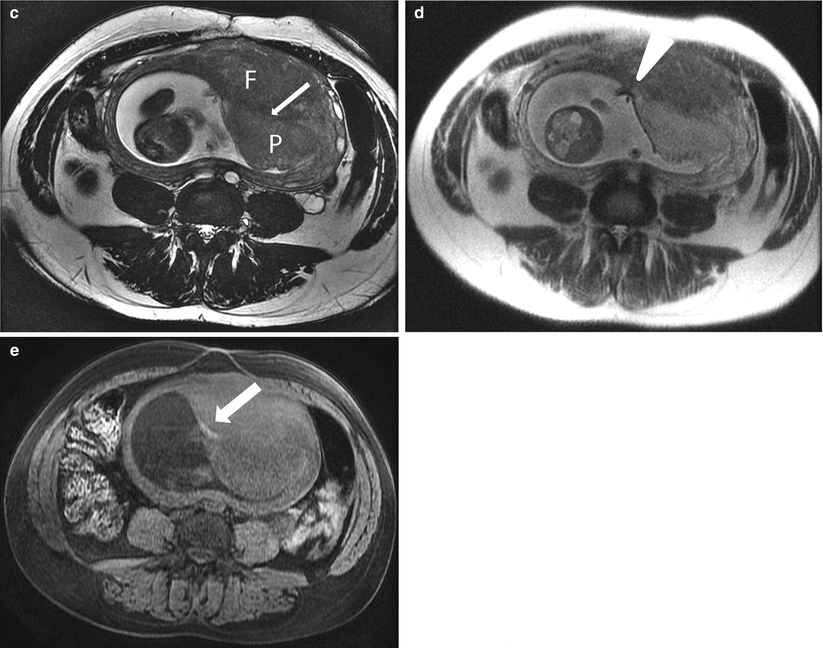

Fig. 13.7
Uterine leiomyoma in a 39-year-old at 32 weeks gestation referred for abdominopelvic MRI. Axial T2-weighted RARE image demonstrates a well-circumscribed hypointense mass in the myometrium (arrows). This mass persisted throughout the exam. Findings were consistent with a myometrial leiomyoma. The placenta (“P”) has implanted in proximity to the leiomyoma. The anterior right lateral placenta itself appears normal and remains smooth in contour with minimal development of heterogeneity in the parenchymal signal, which can be seen as the placenta matures in the third trimester. Prominent periuterine and retroplacental vessels are noted (black arrowheads), not uncommon with advancing parity


Fig. 13.8
Uterine leiomyoma in a 41-year-old at 21 weeks gestation referred for evaluation of uterine mass. Sagittal balanced steady-state free precession (a) and T2-weighted (b) images of the maternal uterus demonstrate a large persistent myometrial mass (“F”) consistent with a leiomyoma. The placenta (“P”) has implanted on the mass (thin arrows, a and c). Axial balanced (c), T2-weighted (d), and T1-weighted (e) images show the marginal cord insertion of the placenta (arrowhead), likely the result of trophotropism as the placenta sought better vascularity away from the leiomyoma. A small crescentic area of T1 shortening (arrow) is also identified in proximity to the cord insertion, consistent with a small marginal hematoma. Localizing this signal is important as flow artifacts within the umbilical cord on GRE images could cause a similar appearance
13.3 Placental Hematomas
Placental hematomas are categorized as preplacental when on the fetal side or retroplacental when on the maternal side. When the hematoma insinuates along the edge of the placenta, it can be described as marginal, while intraplacental hematomas arise within the parenchyma of the placenta itself. On ultrasound, hematomas can be isoechoic to placenta in the subacute phase but are typically hypoechoic or anechoic when acute or chronic. Hematomas lack internal vascularity on color Doppler evaluation. On MRI, hematomas have variable signal intensity, often with areas of T1 shortening present. Hematomas demonstrate restricted diffusion.
Placental abruption is a clinical diagnosis consisting of acute-onset vaginal bleeding, abdominal pain, uterine contraction and tenderness, and non-reassuring fetal heart tracings. The clinical suspicion of abruption is considered a medical emergency because it suggests an acute maternal arterial retroplacental hemorrhage, and management is based on clinical criteria rather than imaging findings. Although MRI would hypothetically be more sensitive for subacute bleeding than ultrasound, it is unlikely to change management, which is based upon clinical features [17, 18]. Areas of hemorrhage in asymptomatic women are felt to result from venous bleeding of a remote nature and treatment would typically be expectant management. Evidence of prior hemorrhage is not an uncommon finding in pregnancy, and when identified, it should be described in detail including multidimensional measurements, location, and proximity to the umbilical cord insertion and cervix. Obstetricians will use this information to determine proper clinical and sonographic follow-up intervals to assess for fetal growth, maternal anemia, and stress (Figs. 13.9, 13.10, and 13.11).
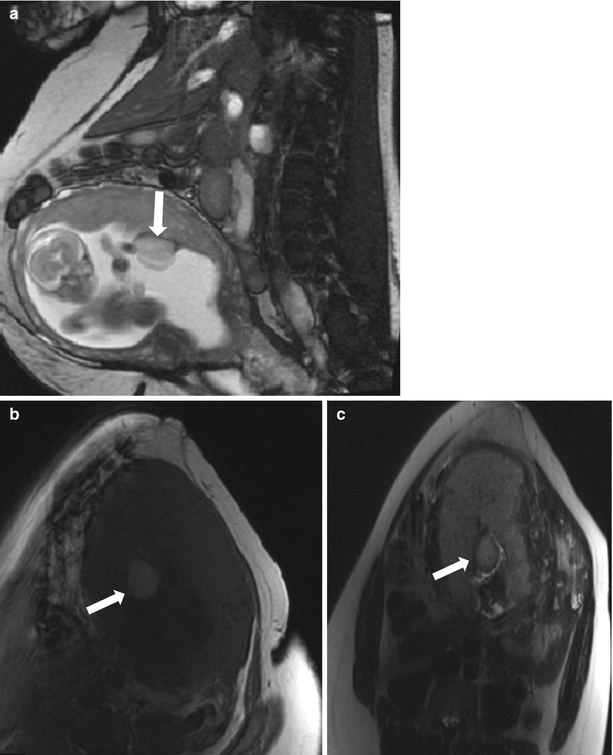
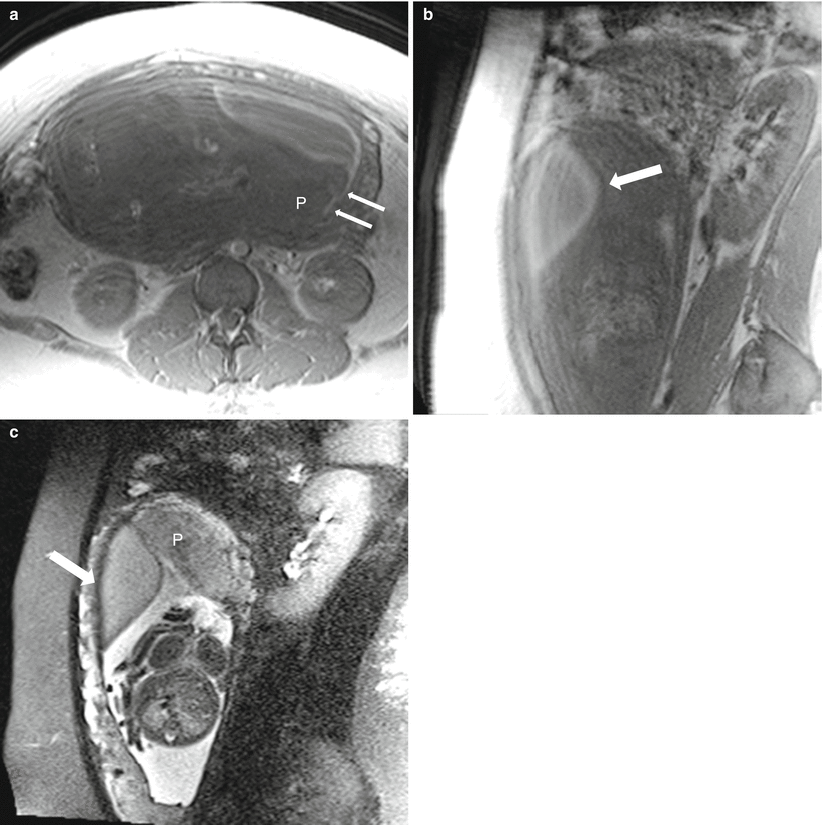
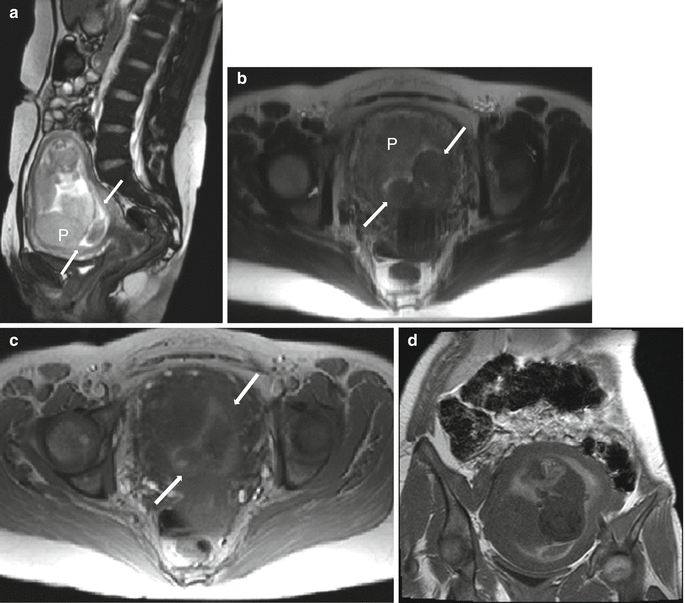

Fig. 13.9
Small preplacental hematoma in a 36-year-old G4P3 at 21 weeks gestation referred for evaluation of a fetal multicystic dysplastic kidney. On sagittal balanced steady-state free precession sequence (a), a mass was identified along the placenta (arrow, a, b). Subsequent axial T1-weighted (b) and T2-weighted (c) images demonstrated signal characteristics consistent with a small subacute hematoma. The location of the hematoma, along the fetal surface of the placenta, is described as preplacental. Relationship to the placental umbilical cord insertion should be offered and follow-up imaging with ultrasound recommended

Fig. 13.10
A 21-year-old G3P2 at 25 weeks gestation with dichorionic twins. Subacute retromembranous hematoma of twin B confirmed at cesarean hysterectomy for placental invasion of twin A (see Fig. 13.15). Axial (a) and sagittal (b) T1-weighted images demonstrate a high-signal intensity lenticular collection (arrow) consistent with blood beneath the fetal membranes on the maternal left. A small amount of hematoma (thin arrows) extends under the placenta (“P”). Sagittal (c) T2-weighted image through the same level as image (b) demonstrates peripheral decreased signal intensity in the hematoma (arrow) and the relationship of the hematoma to the fundal placenta of twin B

Fig. 13.11
Retroplacental hematoma in a 35-year-old at 19 weeks gestation with oligohydramnios and vaginal bleeding. Sagittal balanced steady-state free precession image (a) demonstrates a complete placenta previa (“P”) and heterogeneous material (thin arrows) beneath the placenta, covering the internal os. Axial T2- (b) and T1-weighted (c) images demonstrate the heterogeneous material (thin arrows) which has signal characteristics consistent with acute/subacute blood. Coronal T1-weighted image (d) demonstrates the small volume fluid surrounding the fetus is hyperintense, consistent with intra-amniotic hemorrhage
13.4 Placental Invasion
The spectrum of placental invasion, accreta, increta, and percreta, involves trophoblastic invasion beyond the decidua basalis with chorionic villi attaching to the myometrium, invading into the myometrium, or penetrating beyond the uterine serosa, respectively [7]. The risk of maternal death in the setting of placental invasion has been reported to be up to 7 % [19]. Additionally, over half of women will require intensive care unit admission and/or massive transfusion protocols, and up to 40 % will have postoperative complications [20].
When discussing placental invasion, however, it is important to note that postpartum pathological evaluation can be limited, and it is possible that histopathological sampling error may not identify invasion [21]. In one series of 653 women with previous cesarean delivery, 15 patients with previa required cesarean hysterectomy for bleeding complications, while only 9 had pathological confirmation of placental invasion [5]. Truly, invasive placentas are histologically different from noninvasive placentas [5], yet, for many purposes, the surgeon’s perspective may be more important than the final histological diagnosis. In practice, we develop stronger interpretive ability for placental invasion by receiving feedback from our referring obstetric and gynecologic surgeons who use their surgical determination of depth of placental invasion (accreta, increta, or percreta), as it often has the greatest clinical relevance [22]. Differentiating a focal area of placenta accreta that can be manually extracted from deep myometrial invasion or percreta that requires cesarean hysterectomy is a challenging task but an excellent goal of MR imaging (Figs. 13.12, 13.13, and 13.14).