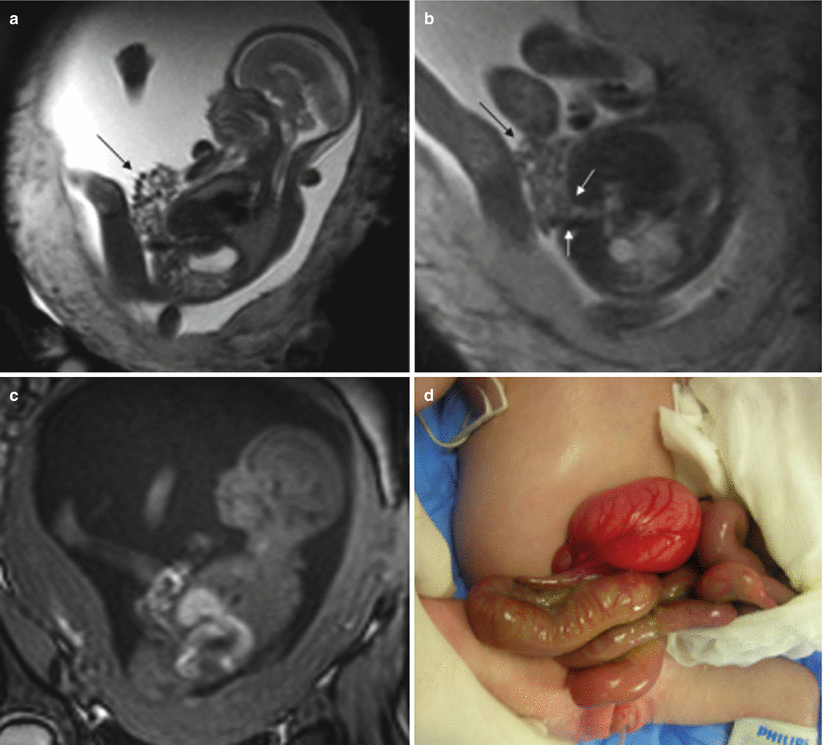
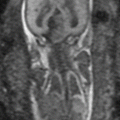
Fig. 11.1
Normal fetal abdominal and pelvic anatomy. Fetus at 32 weeks’ gestation. (a) Coronal and (b) sagittal T2-weighted and corresponding (c) coronal and (d) sagittal T1-weighted images. Stomach (long arrow in a, c), small intestine (short arrow in a, c), colon (thick arrow in a, c, d), rectum (dotted arrow in d), liver (arrowhead in a and c), gallbladder (open arrow in a, b), and bladder (asterisks in a, b). (e) Coronal T1-weighted image and (f) maximum intensity projection (MIP) that allows meconium-based colonography
On T2-weighted images, fetal kidneys are identified as oval structures of intermediate signal intensity on both sides of the spine. Due to the presence of urine, the renal pelvis and calyces are depicted as high-signal structures in the middle of the kidneys. Fetal ureters are not normally seen on T2-weighted images unless dilated, when they are seen as high-signal tubular structures. T1-weighted sequences may help to differentiate dilated ureters from bowel loops, as dilated ureters are seen as low-signal tubular structures, whereas distal bowel loops are hyperintense [3]. The bladder is seen as a fluid-filled round-to-oval structure in the anterior part of the pelvis. It is normal to see changes in bladder volume during MR examination due to fetal micturition. The urethra cannot be seen, but the external genitalia are easily recognized (Fig. 11.2).
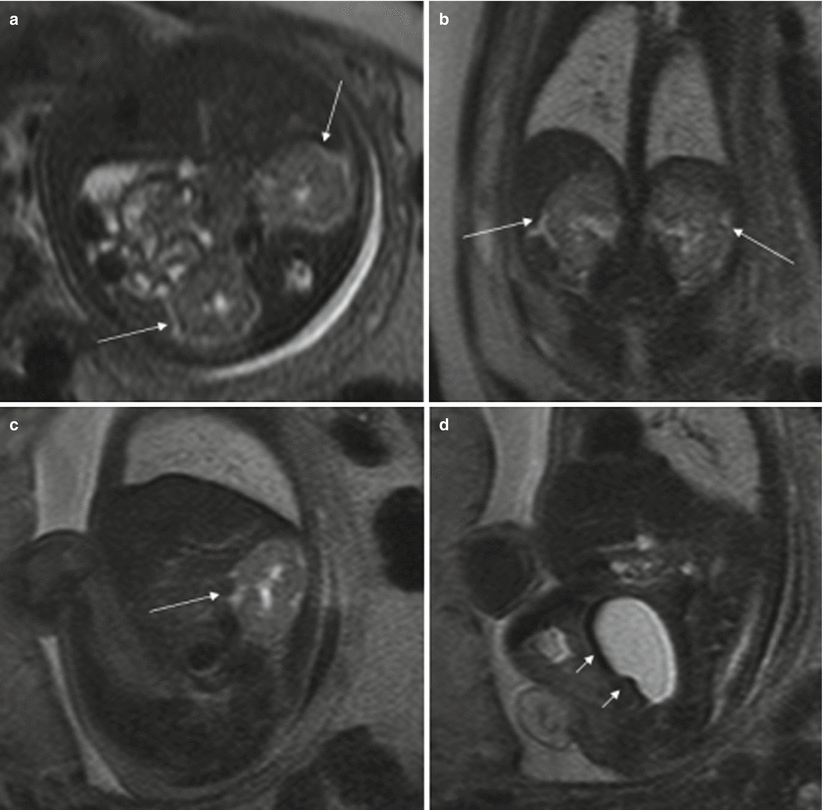

Fig. 11.2
Normal fetal abdominal and pelvic anatomy. Fetus at 31 weeks’ gestation. Fetal axial (a), coronal (b), and sagittal (c, d) T2-weighted images. The fetal kidneys are identified as intermediate signal structures on both sides of the spine (long arrows). The renal calyces and pelvis have high signal intensity due to urine. The fetal bladder is seen as a round-to-oval, high-signal structure in the pelvis (short arrows in d)
The peritoneal cavity, a virtual cavity, is not usually seen except in the presence of ascites. The abdominal wall is easily identified on T2-weighted sequences in all spatial planes. The umbilical cord and its place of insertion are also easily recognizable, especially in the midline sagittal and axial planes. Thick-slab T2-weighted sequences can be very helpful in the evaluation of the umbilical cord.
11.2 Gastrointestinal Tract Anomalies
11.2.1 Stomach Anomalies
In some anomalies, the stomach is not identified in its normal position. In cases of congenital hiatal hernia or diaphragmatic hernias, the stomach may be seen in the thorax (Fig. 11.3). Identification of the stomach in the right side of the fetus or in a central position indicates situs anomalies (Fig. 11.4).
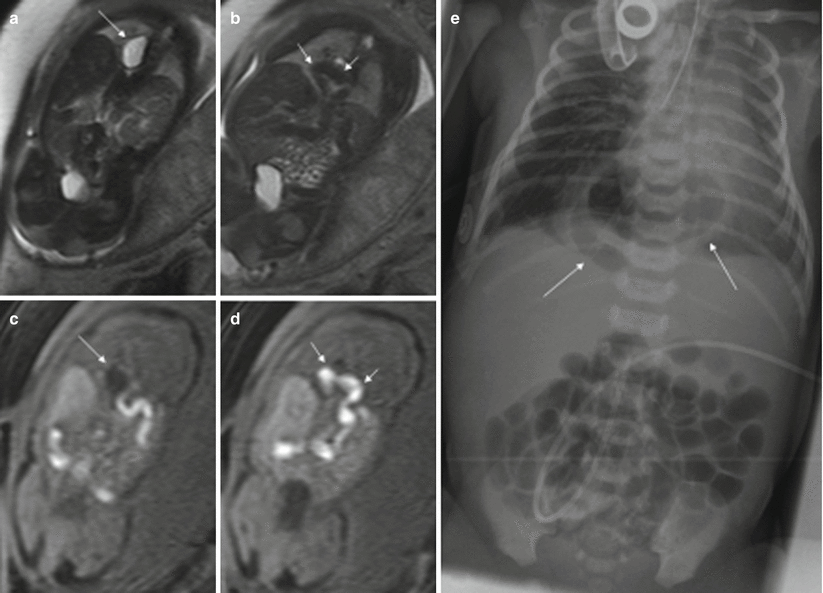
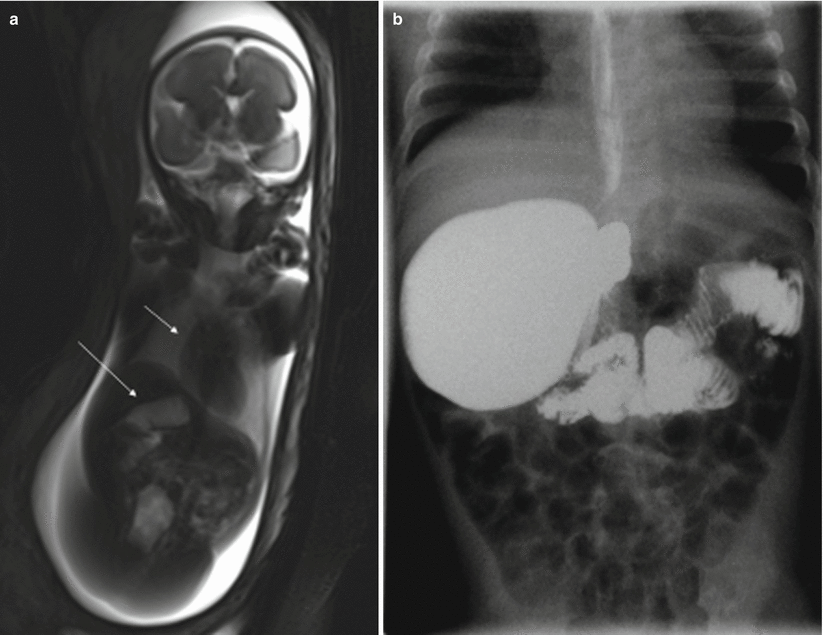

Fig. 11.3
Congenital diaphragmatic hernia. Fetus at 34 weeks’ gestation. (a, b) Coronal T2-weighted and corresponding (c, d) coronal T1-weighted images. The fluid-filled stomach (long arrows in a and c) is seen in the thorax, together with the colon (short arrows in b, d). (e) X-ray after birth shows this anomaly (arrows)

Fig. 11.4
Heterotaxy syndrome. Fetus at 22 weeks’ gestation. (a) Thick-slab coronal T2-weighted image of the fetus shows the fluid-filled stomach in the right upper abdomen (long arrow); the heart is in its normal position (short arrow). (b) Barium studies after birth confirm this anomaly. This child also had polysplenia and malpositioned thoracic and abdominal vessels without heart defects
In cases with polyhydramnios where the stomach is not seen or is very small due to small amounts of gastric fluid, esophageal atresia must be ruled out.
11.2.2 Duodenal Obstruction
Congenital duodenal obstruction can be caused by intestinal malrotation with Ladd’s band, atresia or stenosis, intraluminar diaphragm, annular pancreas, preduodenal portal vein, or volvulus of the middle portion of the intestine, and some fetuses have a combination of these.
In cases of duodenal atresia or marked duodenal obstruction, MR shows dilation of the stomach and the portion of the duodenum proximal to the obstruction as hyperintense structures in T2-weighted sequences [2, 4]. Polyhydramnios is almost always present (Fig. 11.5). When obstruction is due to malrotation with Ladd’s band or volvulus, the loops of the small intestine can be seen lying mostly on the right side of the abdomen while the colon, which is hyperintense on T1-weighted sequences, is on the left (Fig. 11.6). It is important to remember that the stomach and duodenum may be normal sized or even small when the fetus also has esophageal atresia.
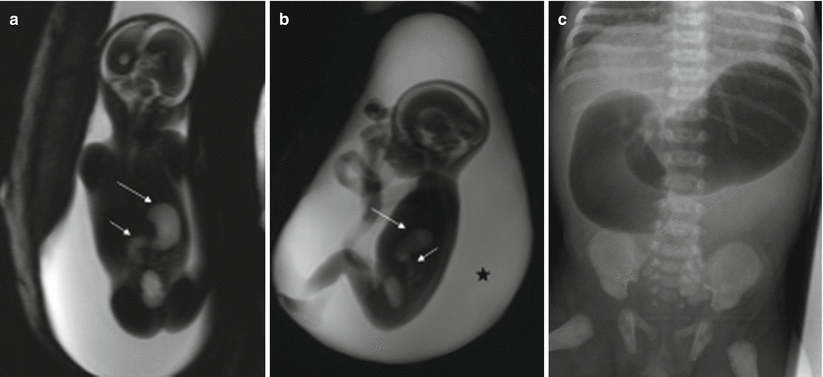
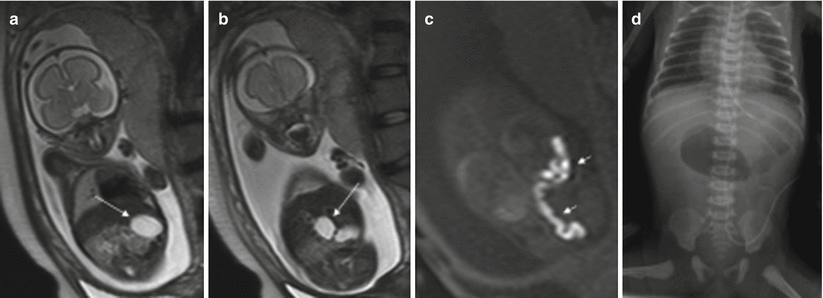

Fig. 11.5
Duodenal atresia. Fetus at 22 weeks’ gestation. Thick-slab coronal (a) and sagittal (b) T2-weighted images of the fetus show gastric (long arrows) and duodenal (short arrows) dilation; polyhydramnios is also present (asterisk). (c) X-ray after birth shows the classical “double bubble” sign that confirms duodenal obstruction, in this case, secondary to duodenal atresia

Fig. 11.6
Intestinal malrotation with Ladd’s band. Fetus at 26 weeks’ gestation. (a, b) Coronal T2- weighted images of the fetus show gastric (arrow in a) and duodenal (arrow in b) dilation. On coronal T1-weighted images (c) the hyperintense colon is seen in the left part of the abdomen (short arrows). (d) X-ray after birth confirms duodenal obstruction. At surgery, intestinal malrotation and Ladd’s band causing duodenal occlusion were found
11.2.3 Small Bowel (Jejunum and Ileum) Atresia/Stenosis
Small bowel atresia presents with dilated bowel loops proximal to the site of stenosis. The most frequent site of atresia is the distal ileum, followed by the proximal jejunum; multiple atresia is not uncommon. Vascular impairment during gut development seems to be the most probable cause of this anomaly [8].
MR detects dilation of the small intestine proximal to the atresia; the stomach and duodenum may also be dilated. The location of obstruction may be suggested by the number of dilated bowel loops. The more proximal the lesion, the more likely it is that the fetus will suffer from polyhydramnios. The signal intensity of the contents of the dilated loops varies and it seems to depend mainly on the site of the obstruction; the more distal the obstruction, the lower the signal intensity on T2-weighted images and the higher the signal intensity on T1-weighted images [2, 4, 9]. The post-atretic bowel may be small in caliber or impossible to identify and generally exhibits abnormal signal intensity [4] (Fig. 11.7).
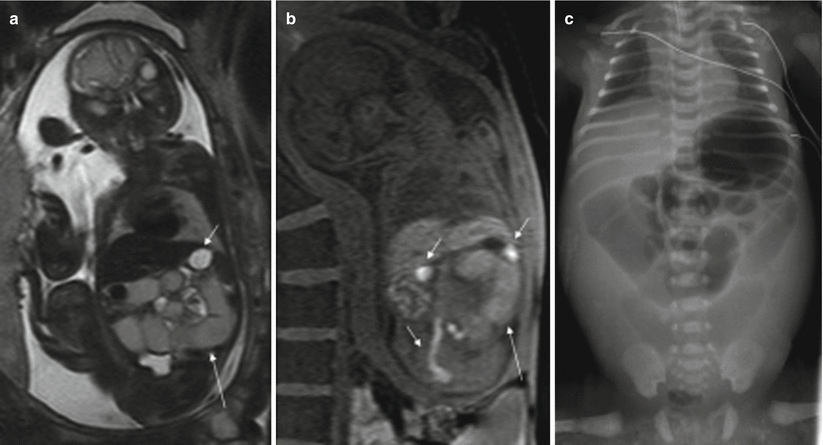

Fig. 11.7
Jejunal atresia. Fetus at 24 weeks’ gestation. (a) Coronal T2-weighted image of the fetus showing dilated proximal small bowel loops of intermediate signal intensity (long arrow); the stomach (short arrow) is not dilated and has higher signal intensity than the small bowel loops. (b) Coronal T1-weighted image shows microcolon (short arrows) and dilated small bowel loops with a higher-than-expected signal intensity (long arrow). (c) X-ray after birth showing dilation of the proximal bowel loops. Jejunal atresia was discovered at surgery
Extraintestinal anomalies are less frequently associated than in duodenal atresia, and the most common associations are with other intestinal anomalies (gastroschisis, intestinal malrotation, meconium ileus) that might be related to the cause of the atresia and to atresias in other locations [5, 10]. A higher incidence of cystic fibrosis has been reported in children with intestinal atresia [11].
Atresia of the small intestine can be very difficult to differentiate from meconium ileus, a condition caused by functional obstruction of the distal ileum by very dense meconium. It is the earliest manifestation of cystic fibrosis. Nearly all newborns with meconium ileus have cystic fibrosis, and 10–15 % of all patients with cystic fibrosis have meconium ileus [12]. The MR findings include dilation of small bowel loops secondary to meconium impaction, microcolon, and polyhydramnios. Ascites may be present whenever the condition is complicated by intestinal perforation. Furthermore, meconium ileus is often associated with gastrointestinal anomalies such as jejunoileal atresia, volvulus, intestinal perforation, and meconium peritonitis.
11.2.4 Meconium Peritonitis
This condition results from intestinal perforation during fetal life. The release of meconium and digestive enzymes into the peritoneal cavity causes chemical peritonitis resulting in an inflammatory reaction and the formation of fibrous tissue that may calcify. Sometimes the inflammatory response seals the perforation spontaneously. In most cases, meconium peritonitis is associated with meconium ileus, intestinal atresia, intestinal volvulus, or intestinal ischemia.
Ascites and dilation of the small intestine can be detected with MR; this is best done using T2-weighted sequences (Fig. 11.8). A meconium pseudocyst may result from a contained perforation. The meconium pseudocyst may exhibit meconium-like signal or high T2 and intermediate T1 signals [4]. Signal intensity can be useful in differentiating meconium pseudocyst from other abdominal cysts. The peritoneal calcifications are difficult to appreciate on MR, as they tend to be small and linear. When seen, they are hypointense in both T1- and T2-weighted sequences. Polyhydramnios may also be present.
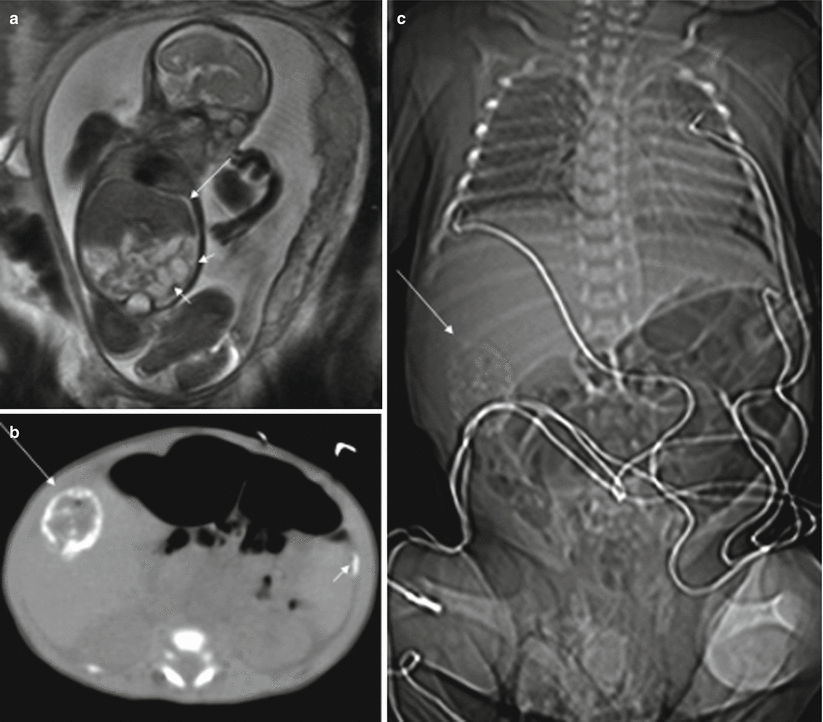

Fig. 11.8
Meconium peritonitis. Fetus at 26 weeks’ gestation. (a) Fetal coronal T2-weighted image showing a small amount of ascites (long arrow) and dilation of bowel loops (short arrows). (b, c) CT after birth shows peritoneal calcifications (short arrow) and a meconium pseudocyst (long arrows) in the right side of the abdomen. This child was asymptomatic
11.2.5 Atresia of the Colon, Anorectal Atresia, and Cloacal Malformations
Atresia of the colon and anorectal atresia are uncommon, and up to 70 % of cases are associated with other anomalies, mostly genitourinary and skeletal, especially of the spine, and with chromosomopathies [13, 14]. As in other atresias, vascular impairment seems the most likely etiology. The MR findings for these entities are not well defined. Intestinal loops above the atresia may be dilated, and meconium accumulated above the atresia may cause high signal intensity on T1-weighted sequences [4, 15] (Fig. 11.9). However, this may not be seen when small bowel atresia is also present.
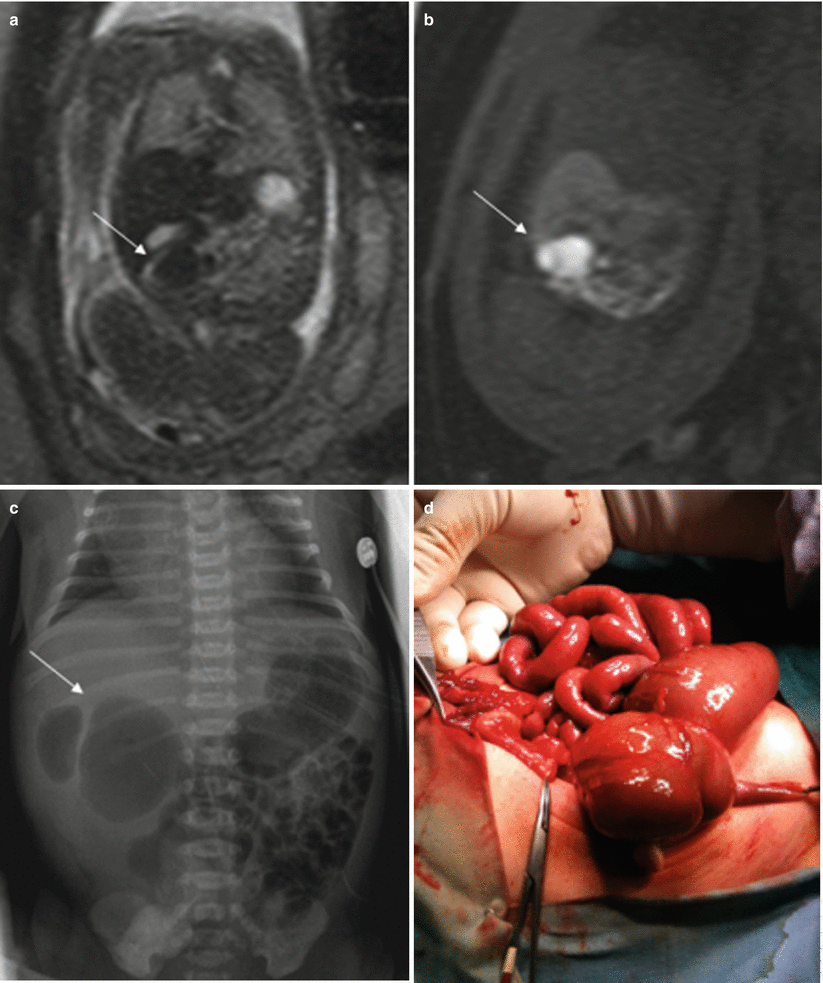

Fig. 11.9
Cecal atresia. Fetus at 22 weeks’ gestation. (a) Fetal coronal T2- and (b) T1-weighted images showing a dilated bowel loop (arrows) in the right hemiabdomen, below the liver; the presence of meconium makes it hypointense on T2- and hyperintense on T1-weighted images. (c) Abdominal X-ray after birth shows a dilated bowel loop (arrow). (d) At surgery, cecal atresia was found
Cloacal malformations represent a spectrum of developmental defects that usually affect female fetuses. In these malformations, the urinary tract, the vagina, and the rectum converge above the level of the perineum, creating a common channel with a single external opening. Cloacal malformations should be suspected in cases with dilation and high position of the distal bowel and abnormalities in the genitourinary system [16]. The typical meconium-filled rectum is not identified in its normal position due to communication between the urinary tract and the rectum (Fig. 11.10).
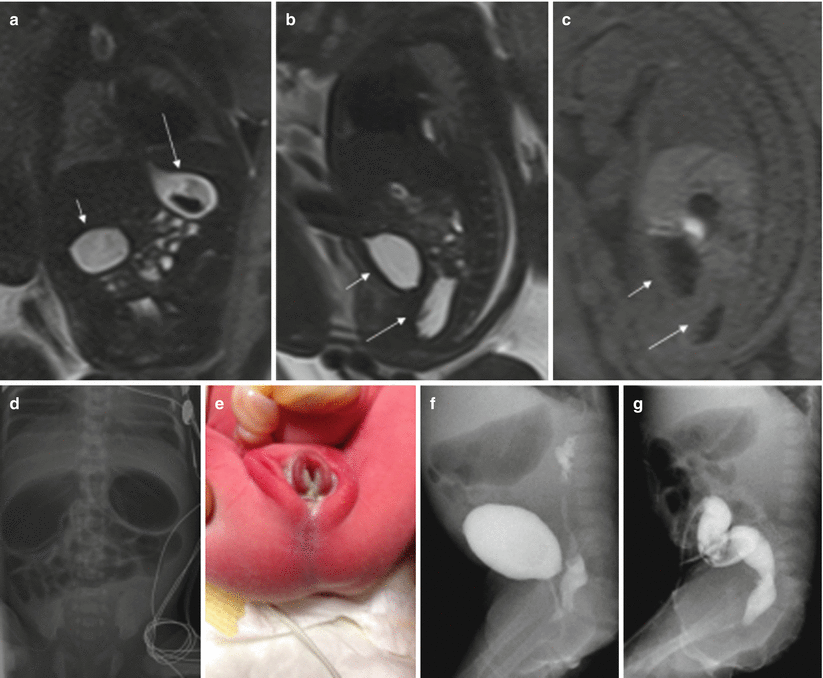

Fig. 11.10
Duodenal obstruction, anal atresia, and cloacal malformation. Fetus at 30 weeks’ gestation. (a) Coronal T2-weighted image of the fetus showing dilation of the stomach (long arrow) and duodenum (short arrow). (b) Sagittal T2- and (c) T1-weighted images of the fetus show the normal fluid-filled bladder (short arrows) and a cystic tubular structure behind it that corresponds to a fluid-filled vagina (long arrows). Note that the normal hyperintense rectum cannot be identified on T1-weighted image. (d) X-ray after birth shows gastric and duodenal dilation, which was due to a duodenal diaphragm. (e) Image of the fetus after birth with anal atresia. (f) On voiding cystography the vagina was filled with contrast. (g) Colonography through the ostomy shows the colon with a cul-de-sac, but no communication with the urinary tract could be seen
11.3 Abdominal Cysts and Masses
There are many abdominal and pelvic cystic lesions or masses that can be detected prenatally, especially during the third trimester. Some originate in solid organs (liver, spleen, adrenal glands, kidneys…) and are easily identified, but others such as enteric duplication cysts, mesenteric cysts, choledochal cysts, urachal cysts, or ovarian cysts may be difficult to differentiate. T1-weighted sequences can be useful for differentiating these anomalies from dilated intestinal loops and meconium pseudocysts.
11.3.1 Enteric Duplication Cyst
Enteric duplications are uncommon congenital abnormalities that can be found at any point along the gastrointestinal tract. They are believed to be caused by defective recanalization of the gut lumen or failure of the notochord to separate from the endoderm.
Intestinal duplication takes place on the mesenteric side of the intestine, and duplications usually do not communicate with the intestinal lumen. The most common site of this anomaly is the distal portion of the ileum, followed by the distal portion of the esophagus and stomach. Although duplications rarely cause intrauterine intestinal occlusion, they may cause intestinal occlusion or abdominal pain after birth, due to volvulus, invagination, or bleeding of the cyst.
On MR they are seen as rounded intraabdominal masses that are hyperintense on T2-weighted sequences and hypointense on T1-weighted sequences [17] (Fig. 11.11). The rest of the intestinal loops tend to be of normal shape, and polyhydramnios is not usually associated.
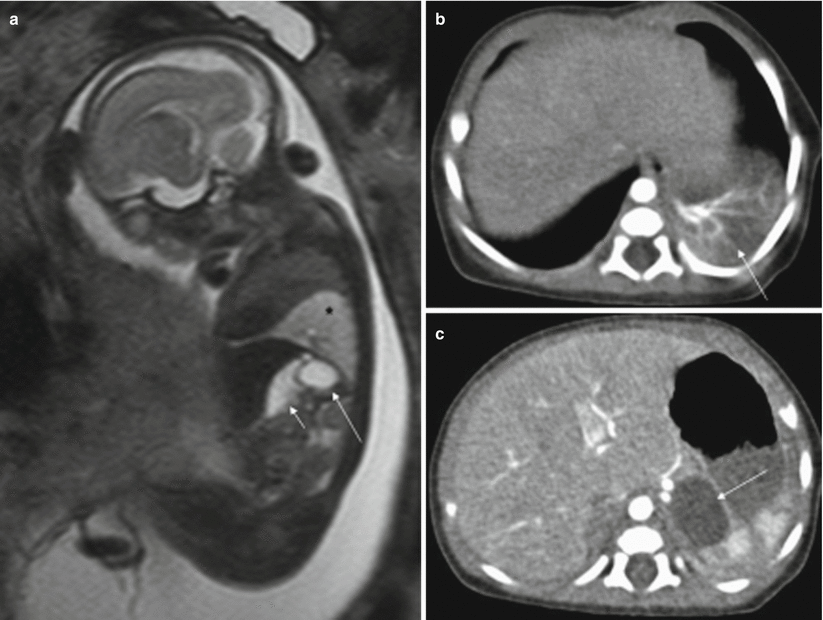

Fig. 11.11
Gastric duplication. Fetus at 23 weeks’ gestation. (a) Fetal sagittal T2-weighted image showing a hyperintense round structure (long arrow) behind the stomach (short arrow). This fetus also had a hyperintense mass in the left lung suggestive of bronchopulmonary sequestration (asterisk). (b, c) CT scan after birth confirms bronchopulmonary sequestration (arrow in b) and gastric duplication (arrow in c)
11.3.2 Mesenteric Cyst
Mesenteric cysts are considered lymphatic anomalies, and they are not normally associated with other fetal anomalies. They are generally single unilocular cysts, although they sometimes can be multilocular. Mesenteric cysts are frequently located in the mid-abdomen within the mesentery, omentum, or retroperitoneum. On MR, they are seen as round unilocular fluid-filled structures.
11.3.3 Ovarian Cyst
Prenatal ovarian cysts are common and are usually detected in the third trimester of pregnancy [20]. They are often unilateral, but can also affect both ovaries. In the fetal period, they may be simple cysts or they can complicate with torsion, bleeding, and/or rupture. Approximately 50 % of these cysts disappear during gestation or the first months after birth [20].
MR shows a round intraabdominal structure that tends to be lateral to the midline. Simple cysts are hyperintense on T2-weighted sequences and hypointense on T1-weighted sequences. When complicated by torsion and/or bleeding, the signal intensity of the cysts is heterogeneous and intracystic components or fluid-fluid levels can be detected due to hemorrhage or detritus [15, 21]. Increased signal intensity on T1-weighted images is indicative of hemorrhage [21] (Fig. 11.12).
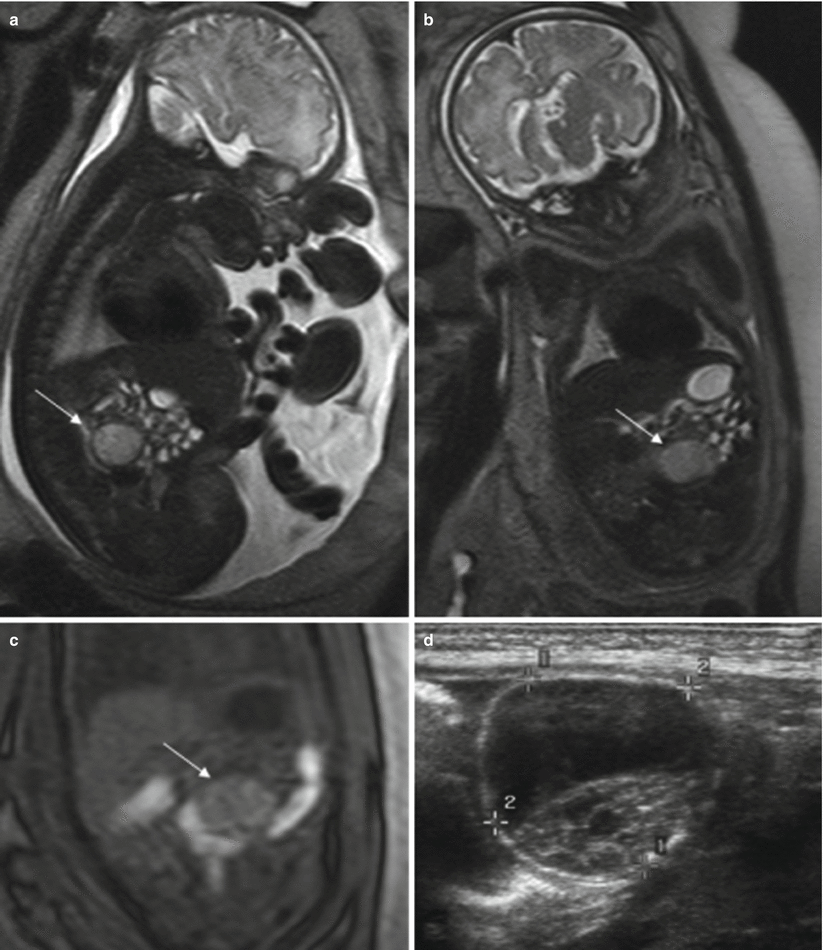

Fig. 11.12
Ovarian cyst. Fetus at 34 weeks’ gestation. Fetal sagittal (a) and coronal (b) T2-weighted images showing a rounded hyperintense structure (arrows) occupying the middle of the abdomen. In the coronal T1-weighted image (c), the lesion has intermediate signal intensity (arrow), raising suspicion of hemorrhage. (d) US after birth shows a complex cystic lesion with debris that corresponded to a complicated ovarian cyst with hemorrhage
Association with other anomalies is uncommon [20].
11.3.4 Liver and Spleen Masses
These lesions are rare in the prenatal period. Masses in the liver and spleen are usually cysts. In the liver, they may appear within the parenchyma or “hang” from the lower edge. They may be single or multiple. On MR they are seen as round structures within the liver or spleen that are hyperintense on T2-weighted sequences [15], unless they contain blood, in which case fluid-fluid levels can be detected in their interior.
Other masses affecting the liver are hemangioma, hemangioendothelioma, hepatoblastoma, hamartoma, and metastases [22] (Fig. 11.13).
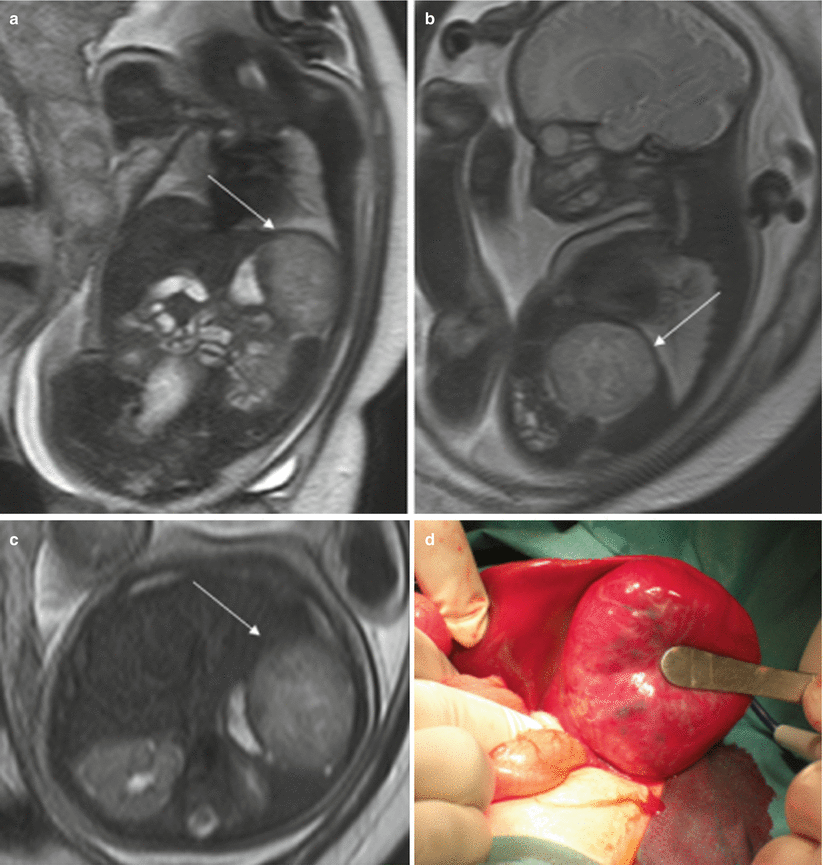

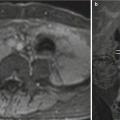
Fig. 11.13
Hepatic hemangioendothelioma. Fetus at 32 weeks’ gestation. Fetal coronal (a), sagittal (b), and axial (c) T2-weighted images showing a mass in the upper left abdomen with intermediate signal intensity (arrows). It is difficult to determine the origin of the mass. (d) Surgery found a hepatic mass hanging from the inferior surface of the left lobe; histology confirmed a hepatic hemangioendothelioma
Hepatosplenomegaly caused by hydrops fetalis, congenital infection, Beckwith-Wiedemann syndrome, or Zellweger syndrome can also be detected prenatally.
Polysplenia can be found in heterotaxy syndromes, but it can be difficult to evaluate with MR [1].
11.3.5 Adrenal Masses
More than 90 % of prenatally diagnosed neuroblastomas arise in the adrenal gland, although they may also be located in the thorax or cervical region [23]. They are normally detected in the right side, almost always in the third trimester [24]. These tumors can disappear before birth, although they can also metastasize, especially to the fetal liver [23, 25]. About half of the tumors are cystic or heterogeneous [23, 26]. The MR appearance of neuroblastoma depends on whether the mass is cystic, solid, or both (Fig. 11.14). Cystic areas are markedly hyperintense on T2-weighted images [27, 28].
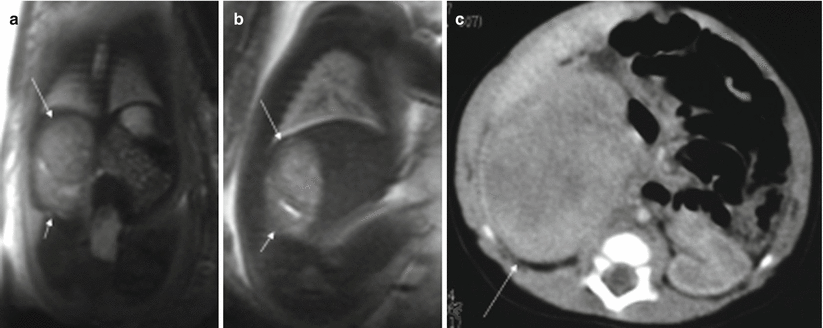

Fig. 11.14
Adrenal neuroblastoma. Fetus at 36 weeks’ gestation. Fetal coronal (a) and sagittal (b) T2-weighted images showing a slightly heterogeneous infradiaphragmatic mass of intermediate signal intensity (long arrows). The mass is above the right kidney, which is displaced posteriorly and downward (short arrows). (c) CT after birth shows this mass (arrow); metaiodobenzylguanidine study after birth was positive
Only half of all adrenal masses are neuroblastomas and should be differentiated from other lesions such as adrenal hemorrhage, renal lesions, or infradiaphragmatic extralobar pulmonary sequestration. Up to 10 % of extralobar sequestrations are infradiaphragmatic and of those more than 90 % of are located on the left side. Unlike neuroblastoma, they may appear in the second trimester [24]. On T2-weighted images they are seen as well-defined high-signal-intensity masses, usually located below the left diaphragm or above the kidney [29, 30]. Hypointense septa may be seen across the lesion [31].
11.4 Abdominal Wall Malformations
Gastroschisis and omphalocele are the two most common congenital abdominal wall defects. Detecting them early during pregnancy allows the best prenatal and postnatal management to be planned.
11.4.1 Gastroschisis
Gastroschisis consists of the herniation of fetal abdominal viscera into the amniotic cavity secondary to a small defect through all the layers of the abdominal wall. Gastroschisis is generally located on the right side of the abdomen with the umbilical cord inserted in its normal position.
Its prevalence is approximately 1.1–5.1 in 10,000 live births with a worldwide increasing trend in recent years [32]. Strong correlations have been found with maternal youth, as well as with drug abuse [33, 34].
MR can detect the evisceration of abdominal structures without difficulties [35–37]. On T2-weighted sequences, eviscerated abdominal structures are clearly visualized floating in the high-signal-intensity amniotic fluid. T1-weighted sequences are useful for determining the position of the large intestine. The axial plane is best to show the defect in the abdominal wall and the position of the umbilical cord (Fig. 11.15).