Fig. 19.1
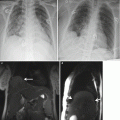
Placental abruption. Axial T1-weighted (a) and T2-weighted (b) MR images show linear low T2-weighted signal intensity signal in the retroplacental region, with corresponding mild increased T1 signal (arrows) consistent with retroplacental hemorrhage. Also seen is the high T1-weighted signal intensity within the uterine cavity consistent with intra-amniotic hemorrhage (*)
USG is often the first modality to begin assessment of vaginal bleeding, and it can show preplacental fluid collection between the placenta and amniotic fluid; jellylike movement of the chorionic plate induced by fetal activity; presence of marginal, subchorionic, or intra-amniotic hematomas; and increased heterogeneous placental thickness (>5 cm in a perpendicular plane) in patients with placental abruption [21]. However, USG has been shown to have low sensitivity (24–53 %) and high specificity (85–96 %) [22, 23]. In patients with a high index of suspicion for abruption and a negative USG evaluation, MR imaging is the imaging modality of choice for further evaluation if necessary. MR imaging, especially with a combination of T1-weighted and diffusion-weighted imaging, is shown to be 100 % sensitive for establishing the diagnosis [22]. Hematomas are seen as well-marginated intrauterine blood collection (Fig. 19.2); the signal intensity of which varies according to chronicity over time. By considering the signal intensity changes on T1-, T2-, and diffusion-weighted images, with special reference to the paramagnetic effects of methemoglobin, it is possible to estimate the age of the bleeding and intrauterine hematomas which can be accurately classified as follows: hyperacute (first few hours, intracellular oxyhemoglobin), acute (1–3 days, intracellular deoxyhemoglobin), early subacute (3–7 days, intracellular methemoglobin), late subacute (>14 days, extracellular methemoglobin), and chronic (>4 weeks, intracellular hemosiderin and ferritin). Further hyperacute/acute hematomas and higher hematoma volume are shown to be associated with clinical deterioration requiring urgent treatment [22].
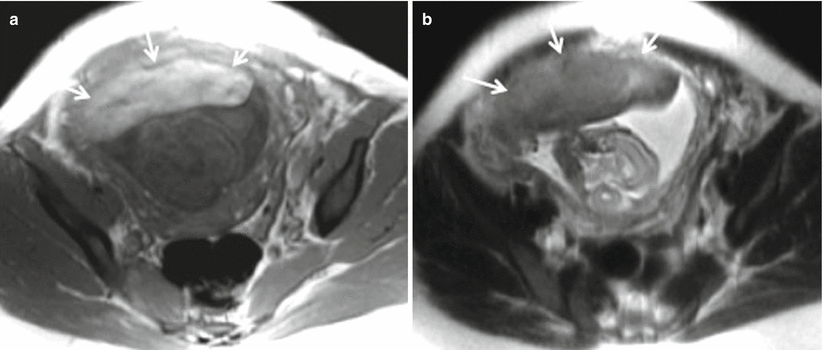

Fig. 19.2
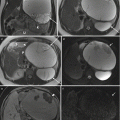
Subchorionic hematoma. Axial T1-weighted (a) and T2-weighted (b) MR images show focal T1 hyperintense, T2 mildly hypointense hematoma in the retroplacental region (arrows) in a pregnant patient presenting with blunt abdominal trauma consistent with subchorionic hematoma
Management of placental abruption depends on the clinical presentation, the gestational age, and the presence or absence of maternal and fetal compromise.
19.3.1.2 Placental Implantation Abnormalities
Placenta previa denotes to placental implantation in the lower uterine segment and can be of complete/central or partial subtypes [24]. The incidence of placenta previa is approximately 0.3–1 % of deliveries [25]. Placenta previa is more frequently seen with multiparity, multiple gestations, advanced maternal age (>40 years), previous cesarean delivery (1.5- to 5-fold risk), previous abortions, and previous placenta previa [26]. Placenta previa is risk factor for placental abruption, hemorrhage, fetal malpresentation, fetal malformations, fetal growth restriction, preterm births, cervical outlet obstruction, and need for cesarean delivery or hysterectomy. Abnormal cord insertion, circumvallate placenta, and placenta accreta (occurs in 3–5 % of patients with placenta previa) also frequently coexist with placenta previa. Clinically, painless bright red vaginal bleeding in the second or third trimester is the hallmark clinical presentation of placenta previa (seen in 70 % cases). Overall, placenta previa can increase perinatal mortality by up to 3–4 times compared with that of normal pregnancy.
On imaging, the diagnosis is established by showing placenta partially or completely covering the internal os (Fig. 19.3). USG is usually sufficient in showing placental position well. MR imaging can serve as a problem-solving tool, because it accurately depicts the abnormality, including a posterior placenta previa, and depicts coexistent abnormalities, including placenta accreta, percreta, or placental abruption which can be challenging on USG (Figs. 19.4 and 19.5). It is important to consider the gestational age at the time of diagnosis because most (>90 %) placenta previas diagnosed at 20 weeks of gestation resolve by term.
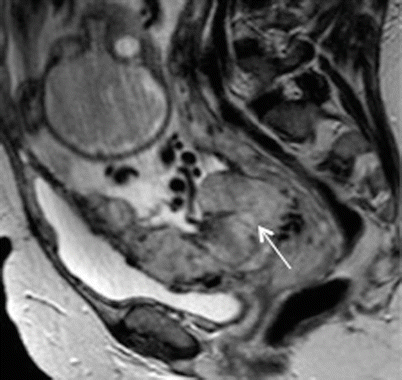
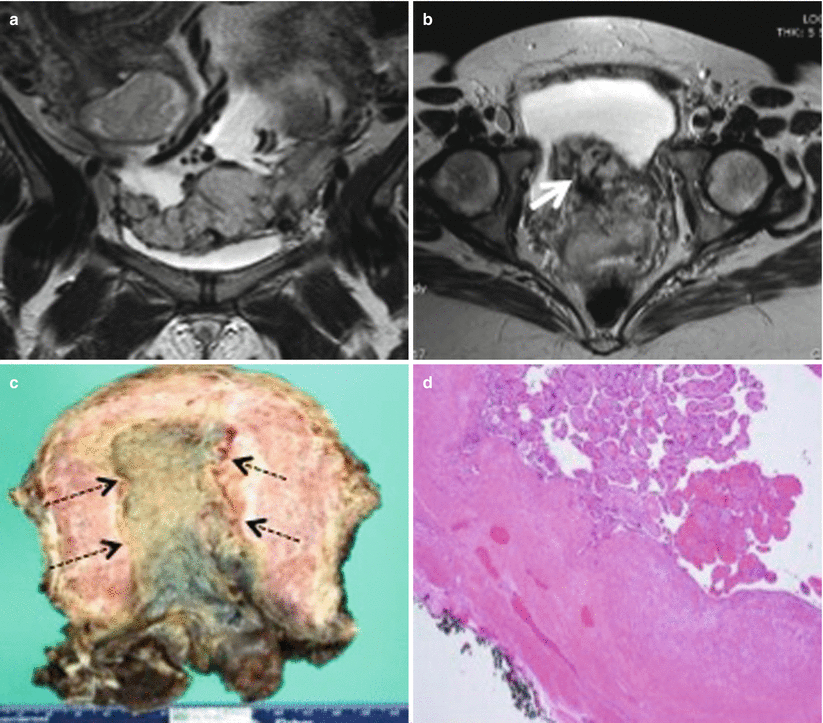
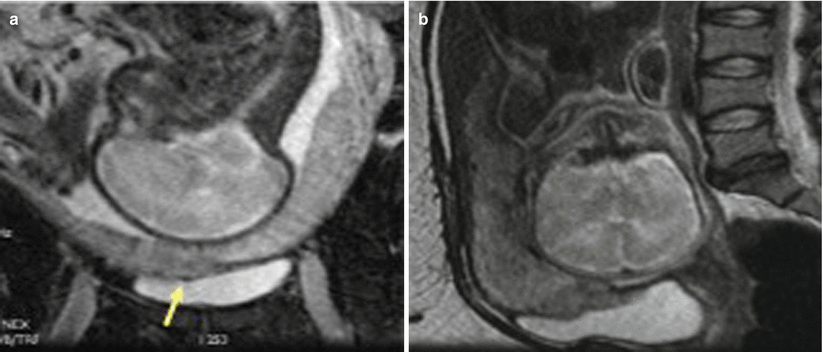

Fig. 19.3
Complete placenta previa. Sagittal single-shot fast spin-echo image of a 30-year-old woman shows the placenta completely covering the internal cervical os (arrow)

Fig. 19.4
Placenta previa with placenta increta. Coronal (a) and axial (b) T2-weighted MR images show loss of normal pear shape of the uterus with randomly distributed dark intraplacental bands anteroinferiorly in low-lying placenta (thick white arrow), raising the suspicion for placenta increta. (c) Gross pathology specimen shows adherent placental tissue (black dotted arrows) to the myometrium. (d) Histopathology slide of the same confirms third trimester villi at the endometrial surface invading the myometrium without intervening decidua

Fig. 19.5
Placenta previa with placenta percreta. Coronal (a) and sagittal (b) T2-weighted MR images show invasion of the placenta beyond the contour of the myometrium into the urinary bladder (arrows) consistent with placenta percreta. There is loss of normal T2 hypointense signal of the dome of the urinary bladder at the site of placental invasion (arrowhead)
Management of placenta previa involves careful maternal and fetal monitoring until term or fetal maturity and an elective cesarean delivery. Vaginal delivery is advocated in placenta previa only if the os-placental distance is more than 2 cm at 35 weeks of gestation.
19.3.1.3 Placental Adhesive Disorders
Placental adhesive disorder results from abnormality in decidua basalis with resultant direct invasion of chorionic villi to myometrium. Depending on extent of placental adherence and myometrial penetration, it can be further categorized as placenta accreta (only adhesion, no myometrial invasion), increta (penetrates myometrium without breaching serosa), and percreta (penetrates serosa and adjacent structures) (Fig. 19.6). Abnormal placentation occurs from 1 in 2500 to 1 in 500 pregnancies [27, 28]. Prior history of cesarean delivery, uterine surgery, and placenta previa are risk factors for abnormal placentation [28, 29].
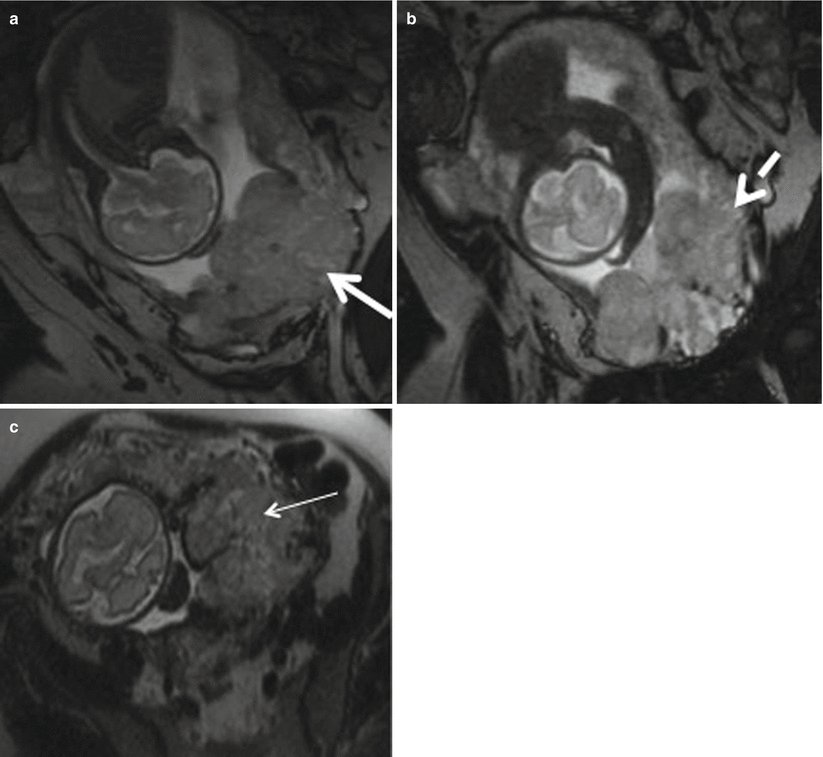

Fig. 19.6
Placenta previa with percreta. (a, b) Coronal T2-weighted MR images showing complete placenta previa (thick white arrow), thickened placenta (white dotted arrow), and bulging of lower uterine segment. (c) Axial T2-weighted MR image shows marked heterogeneity (thin white arrow) of the placenta
Imaging can play vital role in early recognition of these placental disorders that can result in postpartum hemorrhage and/or retained products of conception. Both USG and MRI are complimentary to each other in diagnosing placental implantation abnormalities with reported sensitivity and specificity of MRI in literature ranging from 72–90 % and 81–94 % [30–32]. The normal myometrium has a trilayered appearance on T2-weighted images. The middle layer is a heterogeneously hyperintense vascular layer, with thinner low-signal-intensity muscle layers on either side. Variety of imaging findings can suggest abnormal placentation, which includes myometrial thinning, interruption of the normal layered appearance of the myometrium, lobulated myometrial-placental margin, dark intraplacental bands, abnormal placental vascularity, outward bulging of the placenta with deformity or disruption of the smooth outer uterine contour (Fig. 19.7), and widening of lower uterine segment with hourglass uterine configuration [22, 24, 33, 34]. Treatment is cesarean section and possible cesarean hysterectomy.
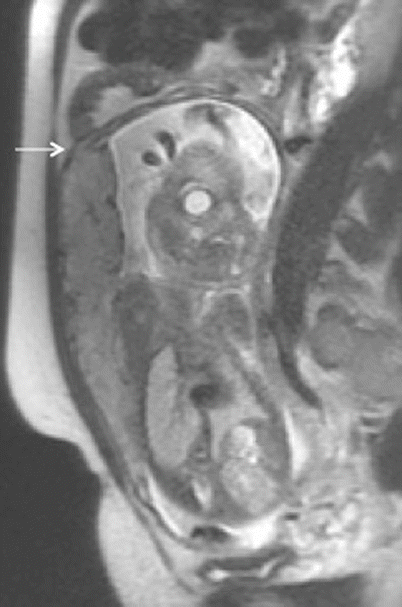

Fig. 19.7
Placenta percreta. Sagittal T2-weighted MR image showing small focus of extra-myometrial-placental extension at the superior and anterior aspect of myometrial wall (arrow)
19.3.1.4 Placental Neoplasms
Placental origin tumors mostly comprise of those arising from trophoblastic tissue. Discussion of other rare placental neoplasm is beyond the scope of this chapter.
Gestational trophoblastic disease (GTD) embraces gamut of disease arising from uninhibited growth of placental trophoblastic tissue, with a spectrum of severity extending from premalignant hydatidiform mole to malignant invasive mole, choriocarcinoma, placental-site trophoblastic tumor, and epithelioid trophoblastic tumor. Hydatidiform mole, usually referred to as molar pregnancy, accounts for 80 % of all GTDs [35]. Hydatidiform mole is estimated to occur in 0.6–1.1 per 1000 pregnancies in North America [36]. Choriocarcinoma is rare with an estimated incidence of 1 in 20,000–40,000 pregnancies [36]. Approximately 50 % of choriocarcinomas arise from molar pregnancies, 25 % from term or preterm pregnancies, and the remainder from pregnancy termination [37]. Risk factors include Asian race, history of oral contraceptive use, prior molar pregnancy, and maternal age (both <20 and >40 years) [38, 39].
Hydatidiform Mole
Hydatidiform moles are classified into two subtypes: complete and partial with distinctive histologic, clinical, imaging, and genetic features [35]. Complete moles result from fertilization of an empty ovum with subsequent duplication of the paternal chromosomes. Gross pathological appearance of the trophoblastic tissue has been classically described as “cluster of grapes” which on USG gives “snowstorm appearance” [40]. No identifiable fetal tissue is formed. Hyperstimulation of the ovaries due to excessive production of β-hCG by abnormal trophoblastic tissue results in formation of theca lutein cysts, which can be present in 20–46 % cases [41].
Partial hydatidiform moles are less common and form due to fertilization of a normal ovum by two sperms with resultant chromosomal composition of 69,XXX or 69,XXY [42]. Fetal tissue may be present which can help in differentiating it from complete mole.
USG is often the first imaging modality to assess a patient presenting with amenorrhea and routinely used in first trimester. Most molar pregnancies now present with findings of early pregnancy failure rather than the classic “cluster of grapes” appearance [43]. Ultrasonography is not very sensitive or specific for detection of GTD and identifies less than 50 % of all hydatidiform moles [43, 44]. Overall, the accuracy of ultrasonography in the detection of complete mole is better than that for partial mole and increases after the 16th week of gestation [42]. MR imaging is archetypally not used in routine assessment of hydatidiform moles but can be used to define if there is extension of molar tissue to the myometrium or outside the uterus. MR imaging findings are nonspecific and can imitate the features of RPOC. Moles appear as heterogeneous tissue expanding the endometrial cavity, with mostly low signal intensity on T1-weighted images, high signal intensity on T2-weighted images, and avid enhancement on postcontrast images (Fig. 19.8). Focal areas of hemorrhage and cystic spaces may also be seen [45]. The intact normal myometrium, appearing as hypointense layer surrounding the molar tissue, helps in differentiation from invasive disease [45].
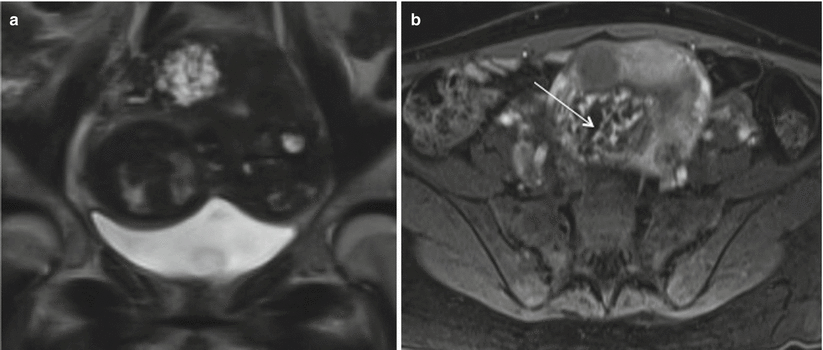

Fig. 19.8
Invasive mole. Coronal T2-weighted (a) and axial T1-weighted gadolinium-enhanced (b) MR images demonstrate a heterogeneous lesion seen in the uterine fundus invading the myometrium, with multiple T2 hyperintense cystic foci, heterogeneous enhancing septae, and multiple nonenhancing cystic spaces Image courtesy- Dr Christine O. Menias (thin arrow). Fetal parts are identified on coronal image
Invasive Mole and Choriocarcinoma
Invasive moles indicate growth of the trophoblastic tissue into and beyond the myometrium. Invasive moles are considered locally invasive neoplasms. Choriocarcinomas can invade locally but are also capable of metastasizing, frequently manifesting with hematogenous metastases. The lung is the most common site (76–87 %) followed by the liver (10 %), brain (10 %), kidney, gastrointestinal tract, and spleen [42, 46, 47]. The vagina is the second most common site of metastasis (30 %), but this occurs through contiguous spread [22, 48].
Invasive moles and choriocarcinomas are mostly similar appearing at imaging. Patients with low b-hCG levels (<500 mIU/mL) often have normal MR imaging findings. Choriocarcinomas tend to show more areas of necrosis, hemorrhage, and solid-enhancing components within the tumor [4]. Choriocarcinoma staging is often performed with CT as it allows detection of distant metastases. MR imaging can have a role in demonstrating myometrial and parametrial invasion and thus aids in the anatomic staging of disease. Choriocarcinoma is often seen as an intrauterine mass with heterogeneous high signal intensity on T2-weighted images and marked enhancement on postcontrast images, findings that reflect the high vascularity of the tumor. Tumor vascularity can be demonstrated by focal signal voids on T1- and T2-weighted images. Myometrial invasion is visible as high-signal-intensity foci within the myometrium causing junctional zone disruption and demonstrate enhancement on postcontrast images. Enhancing parametrial soft tissue is characteristic of local spread. MR imaging can also help detect metastatic disease, particularly within the pelvic organs and lymph nodes. GTDs are treated with primary removal of tumor surgically with or without chemoradiation depending upon stage of disease.
19.3.2 Uterine Complications
19.3.2.1 Rupture/Scar Dehiscence
Uterine rupture accounts for up to 20 % of maternal deaths with estimated overall uterine rupture rate of 1 in 1146 pregnancies (0.07 %). Most uterine ruptures (90 %) are related with a previous cesarean delivery (Fig. 19.9); incidence of uterine rupture with low transverse incision is approximately 0.2–1.5 % and with the classic incision is 4–9 % [49, 50]. Other risk factors for uterine rupture include previous uterine surgeries (Fig. 19.10), multiple gestation, grand multiparity, polyhydramnios, manual removal of the placenta, and other invasive procedures such as dilatation and curettage [3].
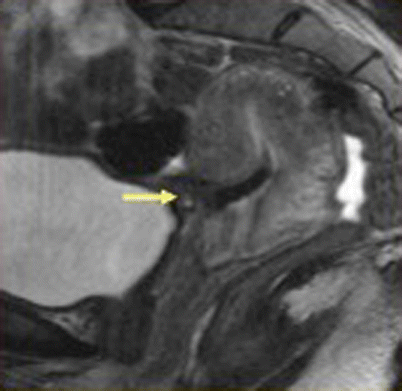
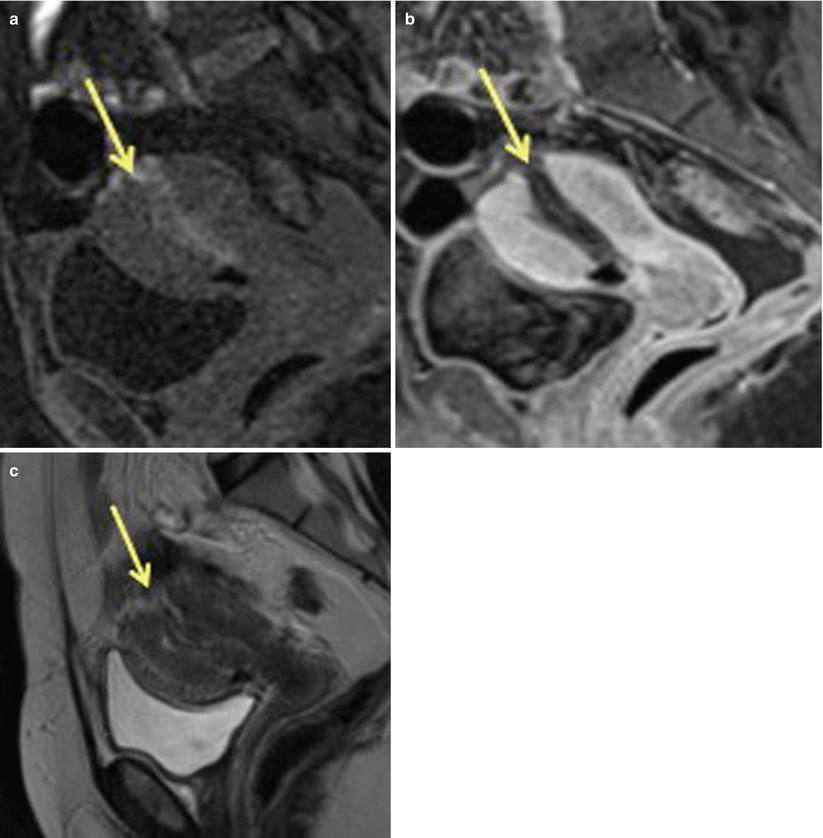

Fig. 19.9
Uterine rupture at the incision site, post-lower segment cesarean section. Sagittal T2-weighted MR image, in a patient with history of previous cesarean delivery who presented with acute pelvic pain and vaginal bleeding, shows a large defect in the lower anterior uterine segment consistent with uterine rupture (arrow)

Fig. 19.10
Uterine rupture at the fundus region post-dilatation and curettage. Sagittal T1 pre- (a) and post- (b) contrast images as well as sagittal T2-weighted MR (c) images show discontinuity in the uterine fundal region (arrow) suggestive of uterine rupture. This MRI was performed after dilatation and curettage of uterine cavity for retained products of conception
The diagnosis is made on clinical symptoms of abdominal pain and vaginal bleeding with shock. Fetal monitoring shows fetal heart rate variations, and tocometer often shows lack of uterine contractions or hyperstimulation without progression of labor raising suspicion of uterine rupture.
Transvaginal USG is often the first imaging study performed which may show intraperitoneal or extraperitoneal hematoma in relation to the uterus, fetal parts in an extrauterine location, intra-amniotic hemorrhage, and focal bulging of membranes through the site of dehiscence [51]. MR imaging may act as a problem-solving tool, showing the presence and extent of rupture in indolent cases with atypical presentation. The difference between the scar dehiscence and uterine rupture is that in scar dehiscence there is separation of an old myometrial scar without penetration of the uterine serosa (Fig. 19.11). The normal appearance of the post-cesarean section incision site on MRI demonstrates signal intensity consistent with subacute hematoma within the myometrium at the presumed incision site; however, overlying endometrial and serosal layers are continuous and no myometrial defects are seen. The primary MR features of uterine dehiscence consist of absence of coaptation of the endometrial or serosal layer with either fluid or blood between the layers of the incision site or low-signal-intensity foci through the incision site suggestive of emphysematous infection.
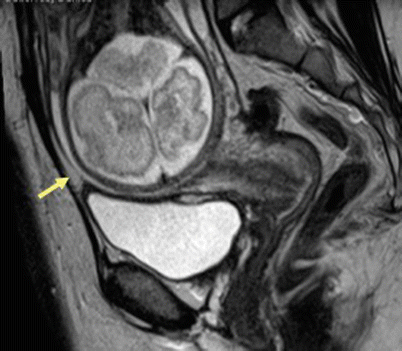

Fig. 19.11
Scar dehiscence. Sagittal T2-weighted MR image in patient with prior history of cesarean section shows thinning of the myometrium in the lower uterine segment without any disruption of serosa (arrow) suggestive of scar dehiscence
Uterine rupture often requires emergent surgery with operative delivery and uterine repair.
Bladder flap hematoma refers to postpartum hematomas that occur beneath the surgically incised and reapproximated peritoneal fold adjacent to the lower uterine segment cesarean delivery incision. Although hematomas in the bladder flap area may be seen normally in the post-cesarean section patient, they are unlikely to exceed 5 cm in size [52]. On MRI and CT, bladder flap hematomas manifest as heterogeneous lesions of varying intensity/density and size extending between the urinary bladder and the lower segment of the uterus (vesicouterine space) [53]. The sagittal images are most helpful when localizing the site of dehiscence and evaluating involvement of adjacent structures such as the bladder. To best demonstrate the myometrial gap, an imaging plane perpendicular to the incision is recommended [52]. If gas is seen within the collection, superimposed infection is suggested. This often requires surgical evacuation if causing symptoms due to mass effect or if it gets infected.
19.3.2.2 Acute Fibroid Degeneration
Leiomyoma are benign smooth muscle neoplasm occurring with prevalence of 11 % during pregnancy [54]. They may be completely asymptomatic even when increased in size due to hormonal changes during pregnancy. Uterine fibroids can be symptomatic in pregnancy because of rapid growth, torsion, or acute red degeneration. Other complications of leiomyomas during pregnancy include pain, bleeding, spontaneous abortion, placental abruption, fetal malposition, and mechanical obstruction of the cervix.
Red degeneration is a hemorrhagic infarction caused by either venous thrombosis or rupture of intratumoral arteries and commonly occurs in the second half of pregnancy or in the puerperium. On MRI, they are seen as sharply marginated, low-signal-intensity masses in submucosal, intramural, or subserosal location. Leiomyomas larger than 3 cm often are heterogeneous due to varying degrees of degeneration. Volume increase >200 cm3 in fibroid is associated with increased rate of complications [55]. Red degeneration shows diffuse or peripheral high signal intensity at T1-weighted images with variable signal intensity at T2-weighted images depending on the degree of intralesional hemorrhage (Fig. 19.12) [10]. These findings correspond with numerous dilated vessels filled with red blood cells at the periphery of the lesion. Management of red degeneration is conservative and involves bed rest, sedation, and analgesia with resolution of symptoms in 7–10 days.
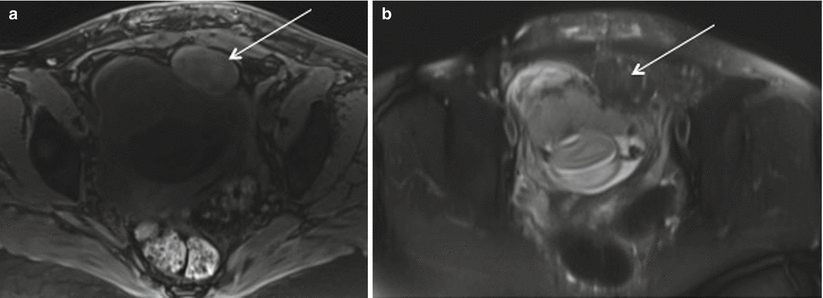

Fig. 19.12
Red degeneration of fibroid. Axial Fat suppressed T1 (a) and T2 (b) images show rounded subserosal fibroid with intrinsic T1 prolongation and T2 shortening in keeping with internal hemorrhage in patient presenting with acute left hemipelvic pain at 20 weeks
19.3.2.3 Retained Product of Conception (RPOC)
RPOC most frequently occurs after second trimester delivery or termination and present with pain, fever, and vaginal bleeding. Ultrasound with color Doppler is the modality of choice, which shows thickened endometrial echo complex (>10 mm) with increased color flow on color Doppler imaging and is suggestive of RPOC. However, there is a high false-positive rate on USG ranging from 17–51 % with blood clot being the most common mimicker of RPOC [56]. Other hypervascular lesions such as uterine arteriovenous malformation (AVM), endometrial polyp, submucosal fibroid, and invasive mole can be difficult to distinguish from RPOC [57]. MRI can be useful tool where RPOC can be seen as variable signal characteristic mass on T2-weighted and precontrast T1-weighted images depending on the degree of hemorrhage and tissue necrosis. It can be seen as variably enhancing endometrial mass with associated uterine disruption making it indistinguishable from gestation trophoblastic disease (Fig. 19.13). However, knowledge about β-hCG levels helps in clinically differentiating these two entities. MR imaging may also be helpful in showing anatomic variants that hinder successful instrumentation of the uterine cavity in patients with suspected retained products of conception [58]. Treatment options for RPOC include uterotonic medications, methotrexate, transarterial embolization, dilation and curettage, and hysteroscopic removal.
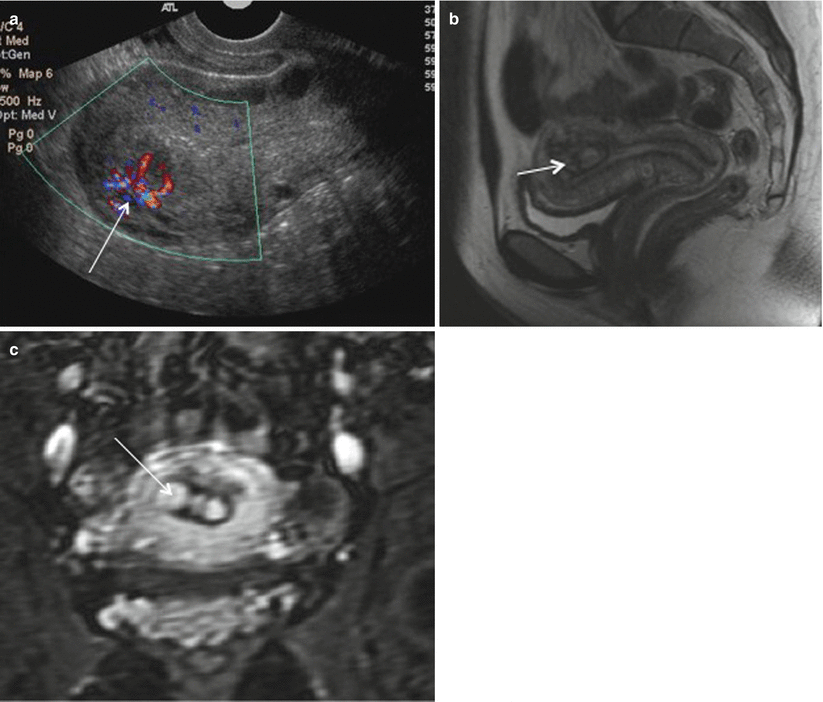

Fig. 19.13
RPOC. Color Doppler (a) ultrasonography images of the uterus show a heterogeneously echogenic endometrial mass with increased vascular flow (arrow). Sagittal T2-weighted MRI (b) and coronal gadolinium-enhanced MR images (c) show heterogeneously enhancing endometrial contents (arrow) consistent with RPOC
19.3.2.4 Endometritis/Wound Infection
Postpartum endometritis denotes to the infection of endometrium or decidua with or without extension into the myometrium and parametrial tissues. It is often polymicrobial (75 %) and arises from ascending infection from vaginal flora. Estimated incidence of endometritis after vaginal delivery is 1–3 %, and the incidence after cesarean delivery is about 20 % [59]. Cesarean delivery, invasive procedure during labor, premature rupture of membranes, prolonged labor, and retained placenta are risk factors for infection during postpartum period. Postpartum endometritis is classified as early (occurring within 48 h) or late (3 days–6 weeks after delivery). If untreated, endometritis may progress to myometritis, pelvic abscess, or septic thrombophlebitis. Endometritis is the clinical diagnosis and may present with fever, lower abdominal pain, foul-smelling vaginal discharge, vaginal bleeding, tachycardia, and leukocytosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree