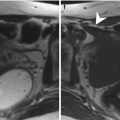
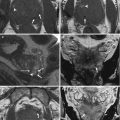
Fig. 1.1
Classification system of Mullerian duct anomalies by the American Fertility Society
1.5.1 Class I Anomalies: Dysgenesis
1.5.2 Class II Anomalies: Unicornuate Uterus
Unicornuate uterus is the result of partial or complete hypoplasia of one Mullerian duct (Fig. 1.1) [11]. Unicornuate uterus may be isolated (35 %) or associated with a contralateral rudimentary horn. The rudimentary horn presents with or without communication to the endometrial cavity and may be associated with or without endometrium, which is also called no cavity rudimentary horn. In patients with cavity non-communicating rudimentary horn, dysmenorrhea and hematometra may occur. Surgical resection either to relieve symptomatic pain or to reduce the risk of potential ectopic pregnancy is justified. As with every obstructed system, the risk of endometriosis is also increased with a non-communicating rudimentary horn. Renal malformations are common with unicornuate uterus and occur mostly on the same side as the rudimentary horn could be found [5].
1.5.3 Class III Anomalies: Uterus Didelphys
Uterus didelphys is a result of complete non-fusion of the Mullerian ducts forming a complete uterine duplication with no communication between each other (Fig. 1.1).
Uterus didelphys may be associated with a longitudinal (75 %) or, more rarely, with a transverse vaginal septum, the latter causing obstructive hematometrocolpos.
Endometriosis as a result of retrograde menstruation may also occur in these conditions. A nonobstructive uterus didelphys is usually asymptomatic [5].
1.5.4 Class IV Anomalies: Bicornuate Uterus
Bicornuate uterus is the result of incomplete fusion of the cranial parts of the Mullerian ducts (Fig. 1.1) [5–9, 12]. Two uterine cavities with normal zonal anatomy can be depicted. The leading imaging feature is a fundal cleft greater than 1 cm of the external uterine contour that helps to distinguish bicornuate uterus from septate uterus.
Extension of the intervening fundal cleft to the internal cervical os characterizes the complete bicornuate uterus with a single cervix (bicornuate, unicollis uterus), whereas variants of partial bicornuate uterus exist if the cleft is of variable length. Bicornuate uterus may be associated with a duplicated cervix (bicornuate bicollis uterus), as well as with a longitudinal vaginal septum that coexists in up to 25 % of bicornuate uterus. Nevertheless, a degree of communication is always present between both uterine cavities.
Still controversial is the need for surgical intervention and this is probably only necessary in specific cases.
A higher rate of cervical incompetence seems to be associated with bicornuate uterus [13].
1.5.5 Class V Anomalies: Septate Uterus
Septate uterus is a result of partial or complete non-regression or the midline uterovaginal septum (Fig. 1.1) [12, 13]. The main imaging feature is that the external contour of the uterine fundus may be either convex or mildly concave (<1 cm) and not with a cleft greater than 1 cm, the latter defining a bicornuate or didelphic uterus.
Septate uterus is the most common Mullerian duct anomaly and is unfortunately associated with the poorest reproductive outcome. Because of different treatment options, septate uterus must be differentiated from bicornuate and didelphic uterus. A widely accepted definition – empirically established during laparoscopy procedures – states that a uterus is septate if the outer contour of the uterine fundus is only mildly concave in the presence of a septum. The cutoff of concavity is 1 cm; deeper concavity is associated with bicornuate uterus and uterus didelphys.
In a complete septate uterus, the septum extends to the external cervical os. In 25 % of septate uteri, the septum extends even further into the upper part of the vagina.
Obstetric outcome seems not to be correlated with the length of the septum. The septum may be composed of muscle or fibrous tissue and is not a reliable means of distinguishing septate and bicornuate uteri.
Resection of the septum by hysteroscopic metroplasty is indicated and may improve the reproductive outcome significantly [5].
1.5.6 Class VI Anomalies: Arcuate Uterus
Arcuate uterus is the result of a near-complete regression of the uterovaginal septum forming a mild and broad saddle-shaped indentation of the fundal endometrium (Fig. 1.1).
Differentiation from bicornuate uterus is based on the complete fundal unification; however, a broad-based septate uterus is difficult to distinguish from an arcuate uterus. There is much controversy as to whether an arcuate uterus should be considered a real anomaly or an anatomic variant. MRI may detect this abnormality, but, typically, it is not clinically significant because arcuate uterus has no significant negative effects on pregnancy outcome [5].
1.5.7 Class VII Anomalies
DES-exposed uterus (Fig. 1.1). DES (synthetic estrogen, diethylstilbestrol, 1948–1971) may induce abnormal myometrial hypertrophy in the fetal uterus forming small T-shaped endometrial cavities, as well as increase the risk of developing a clear cell carcinoma of the vagina.
1.6 Diagnosis of Uterine Anomalies
Diagnosis of uterine anomalies should be based on diagnostic modalities that could determine the anatomical status of the female genital tract on an objective way.
The ideal diagnostic method should provide objective and measurable information on the anatomical status of the uterus in a noninvasive way [1–10, 12–15].
The available diagnostic methods that can be used in the investigation of the patient are as follows.
1.6.1 Gynecological Examination
It should be noticed that gynecological examination is very important in the diagnostic work-up of the patients with congenital malformations.
Vaginal malformations (aplasia, septum) and some cervical malformations could be diagnosed objectively mainly with inspection. Furthermore, palpation through the vagina and/or the rectum (in cases of vaginal aplasia) could provide useful but not always objective information [1].
1.6.2 Two-Dimensional Ultrasound (2D US)
This approach provides objective and, importantly, measurable information for the cervix, the uterine cavity, the uterine wall, and the external contour of the uterus. It is very popular and accessible, noninvasive, but its accuracy highly depends on the experience of the examiner and on the examination methodology followed.
1.6.3 Sonohysterography (SHG)
Compared to 2D US, this method has the additional advantage of offering a better imaging of the uterine cavity, thus enhancing the accuracy in identifying the anatomy of the female genital tract and especially that of the uterus.
With infusion of saline into the endometrial canal, sonohysterography provides improved delineation of the endometrium and internal uterine morphology; however, it shares limitations similar to those of conventional endovaginal US and can only help evaluate patent endometrial canal [17].
1.6.4 Hysterosalpingography (HSG)
HSG is indicated in the early stages of evaluation of the infertile couple. The examination provides a morphologic assessment of the endometrial and endocervical canal and supplies important information regarding tubal patency.
Characterization of uterine anomalies can be difficult; however, there can be considerable overlap in findings, notably with regard to differentiation of a septate from a bicornuate uterus.
The major limitations of the procedure are the ability to characterize only patent canals and the inability to evaluate the external uterine contour adequately.
1.6.5 Three-Dimensional Ultrasound (3D US)
3D US provides an ideal, objective, and measurable representation of the examined organs. It provides information on the cervix, the uterine cavity, the uterine wall, the external contour of the uterus, and the other structures with the exception of tubes. Theoretically, it seems to be an ideal method for the diagnostic approach of the uterus [19–21], but has limited application in clinical practice.
1.6.6 Magnetic Resonance Imaging (MR)
MR imaging has a reported accuracy of up to 100 % in the evaluation of Mullerian duct anomalies.
It is a very useful diagnostic tool, since it can provide clear delineation of internal and external uterine anatomy in multiple imaging planes and, most importantly, reliable depiction of the external uterine contour. Complex anomalies and secondary diagnoses such as endometriosis can often be optimally characterized noninvasively.
1.6.7 Hysteroscopy (HYS)
Hysteroscopy is the gold standard for the examination of the cervical canal and the uterine cavity. However, as it does not provide information on the myometrial layer, hysteroscopy alone could not be used for the differential diagnosis between different groups.
Nowadays, with the use of normal saline as distension medium and the miniaturization of the rigid scopes, hysteroscopy has become a minimally invasive screening tool, well tolerated by the patients and feasible for gynecologists [1].
1.6.8 Laparoscopy and Hysteroscopy (Lap/Hys)
The combined application of these endoscopic techniques is thought to be the gold standard in the investigation of women with congenital malformations and especially the uterine ones.
However, the diagnosis is mainly based on the subjective impression of the clinician performing them, and this is thought to be a limitation in the objective estimation of the anomaly [1].
1.7 Imaging Findings in MDA
Once an MDA is suggested based on evidence from the patient history and physical examination, the next diagnostic step includes different imaging work-ups in order to detect and specify MDA and to guide further treatment options.
Before US and MRI were capable of visualizing MDA with a high accuracy, imaging of MDA was limited to hysterosalpingography (HSG). Since the diagnostic imaging properties of MDA include mainly the configuration of the endometrial cavity and the external uterine contour, HSG is able to depict only certain types of MDA, whereas it fails in other cases and stays nonspecific for precise diagnosis, the latter mainly due to the lack of the visualization of the outer uterine contour. Because of this drawback, HSG did not provide diagnoses with high degrees of confidence, and US and MRI soon began to play a larger role in assessment and treatment of patients. As HSG provides, besides the morphological, also the functional information of tubal patency, it is still used in the primary imaging work-up in case of infertility clarification.
Nowadays, the first imaging modalities in the MDA assessment include pelvic US – transabdominal US (performed with a 2.5- to 5-MHz probe, for evaluation of the entire abdomen, especially for associated renal malformations) and transvaginal US (performed with a 5- to 8-MHz endovaginal probe, for better delineation of the uterus, vagina, and ovaries) – and MRI. Newer techniques, such as 3D US, even further improved the imaging diagnostics by giving better information about the external contour of the uterus and its volume [5, 16, 17].
1.8 Magnetic Resonance Imaging (MRI)
Magnetic resonance imaging (MRI) is today considered standard in the evaluation of MDA and accepted as the leading imaging modality for further surgical planning. MRI provides high-resolution images of the entire uterine anatomy (internal and external contour), as well as of secondary findings like renal malformations.
Among the three major imaging methods, MRI has the best accuracy in the evaluation of uterine anomalies (up to 100 % has been reported) [5, 8, 16, 17, 23, 24].
1.8.1 MRI Technique [11, 17, 25]
Patients should be scheduled to undergo RM examination possibly in the second half of the menstrual cycle, during the follicular and secretory phase when the thickness of the endometrium is increased, thus permitting to better depict the normal zonal anatomy of the uterus. No specific patient preparation is necessary but patients should have an empty urinary bladder; an intramuscular antispasmodic drug could be administered some minutes prior the examination in order to reduce the motion artifacts related to the bowel peristalsis.
At moment, the gold standard for MR imaging of pelvic region is a high-field magnet such as a 1.5 T magnet with a phased array surface coil, but 3 T magnets could be used too [26, 27]. Standard pelvic MR imaging protocols include axial T1-weighted and T2-weighted images. T2-weighted imaging is essential for evaluation of uterine anatomy.
T2 RARE sequences have the best spatial resolution and are those to prefer in order to evaluate the presence of MDA, even if the acquisition time is longer than T2-weighted half-Fourier sequences.
Sagittal sections are best suited to image the uterus point for proper assessment of the uterine fundal contour. In addition it is mandatory to perform axial or coronal oblique sections, depending on uterine lie, parallel to the endometrial cavity resulting in a long-axis view of the uterus.
An additional oblique sequence obtained perpendicular to the cervical results in a short-axis view and allows accurate assessment of the cervix, demonstrating, if present, duplication or septation.
Because this series is pivotal in the evaluation of MDA, it is best performed earlier in the examination, prior to bladder filling, which often displaces consequently the uterus.
A coronal T2-weighted sequence, with a large field of view to enable assessment of the kidneys should be performed in addiction [12, 17].
T1-weighted sequences are mainly helpful to evaluate the presence of any associated pathologies such as ovarian disease; they should be obtained both without and with fat saturation in order to better demonstrate the presence of hemorrhage within the endometrial cavity (hematometra) and/or vagina (hematocolpos).
3D T2-weighted sequences are quick and provide submillimeter section thickness allowing multiplanar reconstruction, thus could be used in pediatric patients to significantly reduce imaging time.
Contrast material is not necessary to evaluate the presence and type of MDA and is not used in standard examination; Gadolinium-enhanced imaging is reserved for assessment of incidentally discovered additional disease.
1.8.2 Specific MRI Findings
1.8.2.1 Class I: Mullerian Agenesis and Hypoplasia
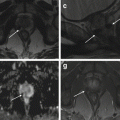
Variable degrees of early failure to form the Mullerian ducts prior to fusion occur in approximately 10 % of uterine congenital anomalies. Complete vaginal agenesis is the common presentation (Mayer-Rokitansky-Küster- Hauser syndrome) (Figs. 1.1 and 1.2) [11, 24–29].
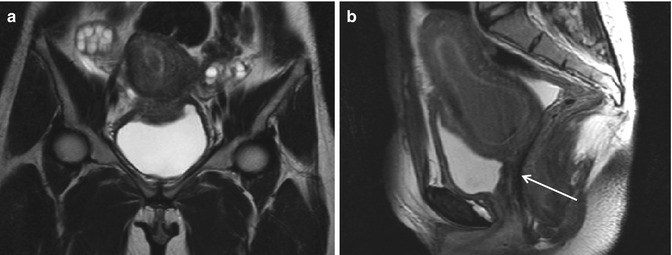

Fig. 1.2




Vaginal agenesis (Ia). Fast spin-echo T2-weighted images on coronal (TR/TE 3310/94) (a) and sagittal plane (TR/TE 4070/94) (b) show normal uterus with cervix and no vaginal cavity (arrow in b)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree