Primary tumor (T)
TX
Primary tumor cannot be assessed (e.g. curettaged or severely regressed melanoma)
T0
No evidence of primary tumor
Tis
Melanoma in situ
T1
Melanomas 1.0 mm or less in thickness
T2
Melanomas 1.01–2.0 mm
T3
Melanomas 2.01–4.0 mm
T4
Melanomas more than 4.0 mm
Note: a and b subcategories of T are assigned based on ulceration and number of mitoses per mm2 as shown below
T classification | Thickness (mm) | Ulceration status/mitoses |
|---|---|---|
T1 | ≤1.0 | (a) w/o ulceration and mitosis <1/mm2 |
(b) with ulceration or mitoses ≥1/mm2 | ||
T2 | 1.01–2.0 | (a) w/o ulceration |
(b) with ulceration | ||
T3 | 2.01–4.0 | (a) w/o ulceration |
(b) with ulceration | ||
T4 | >4.0 | (a) w/o ulceration |
(b) with ulceration |
Regional lymph nodes (N) | |
|---|---|
NX | Patients in whom the regional nodes cannot be assessed (e.g., previously removed for another reason) |
N0 | No regional metastases detected |
N1–3 | Regional metastases based upon the number of metastatic nodes and presence or absence of intralymphatic metastases (in-transit or satellite metastases) |
Note: N1–3 and a–c subcategories assigned as shown below | |
N classification | No. of metastatic nodes | Nodal metastatic mass |
|---|---|---|
N1 | 1 node | (a) Micrometastasisa |
(b) Macrometastasisb | ||
N2 | 2–3 nodes | (a) Micrometastasisa |
(b) Macrometastasisb | ||
(c) In-transit met(s) | ||
Satellite(s) without metastatic nodes | ||
N3 | 4 or more metastatic nodes, or matted nodes, or in-transit met(s)/satellite(s) with metastatic node(s) | |
aMicrometastases are diagnosed after sentinel lymph node biopsy and completion lymphadenectomy (if performed) | ||
bMacrometastases are defined as clinically detectable nodal metastases confirmed by therapeutic lymphadenectomy or when nodal metastasis exhibits gross extracapsular extension | ||
Distant metastasis (M) | |
|---|---|
M0 | No detectable evidence of distant metastases |
M1a | Metastases to skin, subcutaneous or distant lymph nodes |
M1b | Metastases to lung |
M1c | Metastases to all other visceral sites or distant metastases to any site combined with an elevated serum LDH |
Note: Serum LDH is incorporated into the M category as shown below | |
Used with the permission of the American Joint Committee on Cancer (AJCC), Chicago, IL. The original source for this material is the AJCC Cancer Staging Manual Seventh Edition (2010) published by Springer Science and Business Media LLC www.springer.com | |
M classification | Site | Serum LDH |
|---|---|---|
M1a | Distant skin, subcutaneous or nodal mets | Normal |
M1b | Lung metastases | Normal |
M1c | All other visceral metastases Any distant metastasis | Normal Elevated |
Table 24.2
AJCC anatomic stage and prognostic groups for melanoma
Clinical staginga | Pathologic stagingb | ||||||
|---|---|---|---|---|---|---|---|
Group | T category | N category | M category | Group | T category | N category | M category |
Stage 0 | Tis | N0 | M0 | 0 | Tis | N0 | M0 |
Stage IA | T1a | N0 | M0 | IA | T1a | N0 | M0 |
Stage IB | T1b | N0 | M0 | IB | T1b | N0 | M0 |
T2a | N0 | M0 | T2a | N0 | M0 | ||
Stage IIA | T2b | N0 | M0 | IIA | T2b | N0 | M0 |
T3a | N0 | M0 | T3a | N0 | M0 | ||
Stage IIB | T3b | N0 | M0 | IIB | T3b | N0 | M0 |
T4a | N0 | M0 | T4a | N0 | M0 | ||
Stage IIC | T4b | N0 | M0 | IIC | T4b | N0 | M0 |
Stage III | Any T | ≥N1 | M0 | IIIA | T1–4a | N1a | M0 |
T1–4a | N2a | M0 | |||||
IIIB | T1–4b | N1a | M0 | ||||
T1–4b | N2a | M0 | |||||
T1–4a | N1b | M0 | |||||
T1–4a | N2b | M0 | |||||
T1–4a | N2c | M0 | |||||
IIIC | T1–4b | N1b | M0 | ||||
T1–4b | N2b | M0 | |||||
T1–4b | N2c | M0 | |||||
Any T | N3 | M0 | |||||
Stage IV | Any T | Any N | M1 | IV | Any T | Any N | M1 |
Melanoma may metastasize through local extension, via lymphatics prior to first draining lymph node (in-transit metastases) to regional lymph nodes, or systemic metastases to distant sites. AJCC stage I–II melanomas usually develop regional nodal metastases as the initial first site of metastatic disease; of those patients who develop nodal metastases, the majority will also present with satellite/in-transit disease or distant metastases [24]. Apart from lymph nodes, the most common sites for metastatic disease are skin, lung, liver and brain, although almost any site in the body may be affected [24–26]. The patterns of spread of disease are dependent on tumor site and thickness, with approximately one-third of melanomas from the lower extremities metastasize locally to satellite/in-transit metastases, whereas nearly one-third of melanomas from the trunk and upper extremities disseminated directly to distant sites [24]. In contrast, head and neck primary melanomas develop local, regional, and distant metastases in nearly the same proportions (about one-third). Distant metastases were most often observed in men (mainly trunk melanomas), while loco-regional metastases were rather detected in women, who have an increased incidence of lower limb melanomas.
Treatment for Localized Melanoma
Surgical excision is the primary treatment for melanoma. Several randomized, controlled trials have determined appropriate resection margins according to the thickness of the primary melanoma. For melanoma in situ, margins of 0.5–1 cm around the visible lesion or biopsy scar are recommended. Resection margins of 1–2 cm are recommended for 1.0–4.0-mm-thickness primary lesions, and 2–3-cm margins if the primary lesion is >4.0 mm thick.
Regional lymph node spread of melanoma, or in-transit disease, may occur at the time of initial presentation, or usually within 3 years of initial diagnosis. Lymph node resection is usually performed for regional nodal disease. Local radiation treatment may also be performed for regional lymph node metastases, depending on the extent of disease and success of initial surgery, and is also commonly used for in-transit disease.
Prognostic Factors
Patients with melanoma can be stratified into various risk groups: those with stage IA disease who carry a very low risk of recurrence and a 5-year survival of approximately 95%; those with stage IB and IIA disease, who have an intermediate risk and a 5-year survival from 65 to 90%; those with IIB, IIC, and IIIA disease who have a higher risk; and those with stage IIIB and IIIC disease who carry a very high risk of recurrence and a 5-year survival from 45 to 70%.
Over the past 10 years, there has been a debate focused on the optimal diagnostic, prognostic and therapeutic strategies for cutaneous melanoma. It is important to identify and evaluate prognostic factors that can accurately categorize patients into different risk groups for distant metastatic disease. Currently, the most useful prognostic factors in clinical practice for localized melanoma are Breslow thickness, presence of lymph node involvement and ulceration.
Sentinel lymph node biopsy (SLNB) is important for planning therapy, although it has failed to show any benefit in overall survival. There has been no consensus on which patient categories benefit from SLNB. The National Comprehensive Cancer Network recommends that SLNB should be considered for patients with high-risk stage IA melanoma and discussed and offered to patients with stage IB–IIC melanomas [27]. SLNB can identify patients who may benefit from more extensive staging investigations and, based on pathology results, may be suitable for adjuvant therapy [28].
Thickness and ulceration of melanoma are highly correlated with each other, and studies have shown that the incidence of melanoma ulceration rises with increasing tumor thickness, ranging from 6–12.5% for thin melanomas to 63–72.5% for thick (>4.0 mm) lesions. The presence of ulceration is associated with decreased survival in all tumor thickness categories. Thin (<1.0 mm) ulcerated tumors have approximately a 4% decreased 5-year survival rate compared to non-ulcerated tumors. This survival decrement is as high as 22% in thick (>4.0 mm) tumors [21]. Furthermore, the tumor mitotic rate is an additional independent prognostic factor which has emerged as more powerful than ulceration in several studies [29–31]. A better understanding of prognostic factors together with accurate staging in cutaneous melanoma allows the identification of patients whose risk of recurrence is sufficiently high to justify adjuvant systemic treatment.
Adjuvant Therapy
Attempts to reduce the incidence of recurrent melanomas with adjuvant therapy have been studied in more than 100 randomized controlled trials [32, 33]. The role of chemotherapy remains unclear with no tangible benefit in terms of prolonged overall survival. Although the benefit of adjuvant treatment in patients at high risk of recurrence is still disappointing, interferon remains the most active adjuvant agent evaluated to date for high-risk melanoma.
High-dose interferon (IFN) has been evaluated in three large United States cooperative group trials. The data from ECOG 1684, which led to FDA approval of IFN for the treatment of patients with resected stage IIB and stage III melanoma, showed a 5-year relapse-free survival improvement and suggested a statistically significant benefit in overall survival at a median follow-up of 6.9 years [34]. However, at 12.6 years of follow-up, the overall survival was not significantly different between the two groups, even though the relapse-free survival benefit was confirmed. A pooled analysis of three large randomized trials of high-dose IFN confirmed a consistent and significant effect on recurrence-free survival in patients with high-risk resected melanoma, but did not find a statistically significant improvement in overall survival [35]. High-dose IFN is approved in the USA and Canada, but is not currently accepted worldwide due to its considerable toxic effects and to the fact that the benefit occurs in only a minority of the patients at risk. A large phase-III trial (EORTC18991) compared the treatment with pegylated interferon-a2b for up to 5 years with observation in Stage III patients. The results indicated a significant impact on relapse free survival without a significant improvement of distant metastasis-free survival or overall survival [36]. Based on this data, pegylated interferon alfa received approval by the FDA in March 2011 for the adjuvant treatment of stage III disease. Efforts to improve the efficacy and toxicity profiles of interferon have also included the use of lower-dose regimens, and combination with various drugs and vaccines [37–40]. Several phase III trial are currently testing the potential role of new treatments, including the use of immunotherapy (ipilimumab, MAGE 3 vaccine) and BRAF and MEK inhibitors (vemurafenib, dabrafenib, trametinib) in the adjuvant setting.
Appropriate adjuvant treatment for patients with intermediate- or high-risk melanoma remains controversial. The 2010 National Comprehensive Cancer Network (NCCN) guidelines suggest IFN as a category 2B option for patients with stage IIB or greater disease. However decisions about its appropriateness for selected patients should be made on an individual basis. Decisions regarding adjuvant therapy for neoplastic diseases represent a balancing of risk of disease recurrence against the relative value of the agent used in terms of its activity, toxicity, and cost.
Treatment of Metastatic Malignant Melanoma
Approximately 2–5% of new patients with melanoma present with metastatic disease, and 30% of patients initially diagnosed as localized or regional disease develop distant metastasis [41]. The prognosis for patients with advanced visceral metastatic melanoma is particularly poor with 1-year survival rate of only 33% [42, 43]. Until recently, the treatment options for advanced or metastatic melanoma remained very limited. Dacarbazine and interleukin-2 (IL-2) were the only two drugs approved by the US Food and Drug Administration (FDA) for the treatment of advanced melanoma.
Chemotherapy, usually with single-agent dacarbazine (DTIC), has been the standard first line treatment for advanced metastatic melanoma until 2011 despite its limited clinical benefit. In clinical studies, the response rate observed with single-agent DTIC chemotherapy ranges from 15 to 25%, with median response durations of 5–6 months [44]. However, in modern-designed phase III trials that used strict response assessment criteria, the response rates with DTIC did not exceed 12% [45]. Complete responses were rare and short in duration (3–6 months). No multi-agent chemotherapy has yet proved superior to single-agent DTIC chemotherapy.
Temozolomide (TMZ), a prodrug of DTIC, is a novel oral alkylating agent which is characterized by significant central nervous system penetration and relatively little toxicity. Clinical trials have suggested that temozolomide has activity that is equal to that of dacarbazine [46]. Several other chemotherapy drugs have also been tested in melanoma, including the nitrosoureas, vinca alkaloids, platinum analogues and taxanes, but there is no evidence that any of these agents produces better outcomes than dacarbazine. A variety of regimens combining dacarbazine with other cytotoxic agents have shown promising response rates; however, there is no evidence from randomized trials that combination chemotherapy is superior to dacarbazine alone in the treatment of metastatic melanoma [47, 48].
Finally, IL-2 plays a central role in the immune system by modulating the immunologic effects of T lymphocytes, natural killer (NK) cells, B cells and macrophages. High-dose bolus interleukin-2 received FDA approval in 1998 because the regimen led to durable responses in approximately 6% of patients and resulted in overall objective response rates of about 17% [49]. Unfortunately this regimen is associated with many severe toxic effects which have restricted the use of this therapy to highly selected patients being treated in specialized centers. After several decades of negative studies, it is only recently that rapid advances in immunotherapy and the use of drugs targeting the mitogen-activated protein kinase (MAPK) pathway have dramatically improved outcomes in advanced melanoma. In 2011, the US FDA approved two novel therapies for advanced melanoma: a BRAF inhibitor, vemurafenib, and an immune stimulatory agent, ipilimumab, following the positive impact in overall survival shown by randomized phase III trials.
Novel Therapeutic Approaches
Vaccines and Antibodies
The anti-tumor immune response has long been considered to be important in the prognosis of melanoma, and efforts to harness the human immune system to treat cancer have been the focus of clinical development. An improved understanding of the cellular interactions regulating cancer immunity has led to a new era in the treatment of patients with metastatic melanoma. Ipilimumab, a monoclonal antibody binding to the CTLA-4 receptor expressed in activated T cells, was first agent to demonstrate an overall survival benefit in the treatment of advanced melanoma. A phase III study in previously treated metastatic melanoma compared ipilimumab plus a gp100 vaccine versus ipilimumab alone versus the vaccine alone [132]. A more recent phase III study compared dacarbazine with or without ipilimumab in previously untreated melanoma patients [133]. Both studies showed durable responses and a significant improvement in overall survival in the patients who received ipilimumab. The potential value of higher doses of ipilimumab, as well as ipilimumab in combination with other agents is currently being evaluated in clinical trials. A variety of vaccines and antibodies against melanoma antigens have also been studied, with mixed results to date. Vaccines against NY-ESO-1, a cancer-testis antigen, have shown induction of T cell and antibody responses in melanoma patients and some evidence of clinical activity [50]. GD3 (ganglioside) is an attractive target due to its high expression on melanoma cells with limited expression on normal tissues. Antibodies against GD3 have been tested in clinical trials [51, 52]. To date no vaccine therapy has been shown to be effective for the treatment of metastatic disease.
Anti-angiogenic Therapy
Blockade of the VEGF pathway has been achieved by many different approaches, including blocking antibodies targeted against VEGF or its receptors, soluble decoy receptors that prevent VEGF from binding to its normal receptors (VEGF trap), as well as small-molecule inhibitors of the tyrosine kinase activity of the VEGFRs. Bevacizumab is a potent antibody against the VEGF and has been shown to be effective for the treatment of various advanced cancers. Bevacizumab has also been studied in phase II trials in metastatic melanoma [54, 55]. Prolonged disease stabilization and response rates have been demonstrated in one-quarter of metastatic melanoma patients after the addition of bevacizumab to low-dose IFN, and dacarbazine [56]. Further trials of bevacizumab combined with chemotherapy are ongoing in melanoma patients, and numerous other anti-angiogenic agents are currently in clinical trials or in development.
Apoptotic Therapy
The Bcl-2 protein is one of the most important inhibitors of apoptosis, and it is overexpressed in approximately 90% of melanomas. Drug resistance in melanoma has been partially attributed to overexpression of Bcl-2. Oblimersen sodium is a Bcl-2 antisense oligonucleotide that selectively targets Bcl-2 RNA for degradation. Oblimersen has been studied in phase I/II and phase III trials in melanoma patients and has shown significant increases in progression-free survival and response rate when oblimersen was combined with dacarbazine [57]. Ongoing studies are aimed at defining the patients who will respond best to this treatment, including those with low baseline serum LDH levels.
RAF Inhibitors
Earlier attempts to inhibit BRAF in melanoma patients with sorafenib were largely unsuccessful. Despite encouraging phase 2 results reporting disease stabilisation in a few unselected patients, further phase II and III studies showed little anti-tumor activity for single-agent sorafenib and in conjunction with chemotherapy [58, 59, 134, 135]. Recently, two agents have demonstrated significant clinical benefit in melanoma: vemurafenib (PLX4032) and dabrafenib (GSK2118436). Vemurafenib is a selective BRAF inhibitor that targets the V600 mutant forms of the BRAF. A phase 2 trial (BRIM2) involving patients with previously treated BRAF V600E mutant stage IV melanoma showed a response rate of 53%, with a median duration of response of 6.7 months [136]. A subsequent phase 3 randomized trial (BRIM3) of previously untreated patients compared vemurafenib with dacarbazine. This study demonstrated an improvement in progression free survival (6.9 months versus 1.6 months), overall survival (13.6 months versus 9.7 months) and overall response rate (57% versus 8.6%) [137]. A second BRAF inhibitor, Dabrafenib has shown similar results to those with vemurafenib in phase I/II studies [138, 139]. A Phase III randomized trial compared dabrafenib with dacarbazine in previously untreated patients with BRAFV600E-mutated metastatic melanoma. Dabrafenib significantly improved progression-free survival (5.1 months versus 2.7 months) and response rates (53% versus 19%) compared with dacarbazine [140]. Resistance to BRAF inhibitors develops in the majority of patients and can be mediated by a number of different mechanisms occurring along the MAPK pathway (eg, mutations in MEK, NRAS, overexpression of COT) or via bypass signalling pathways (eg, activation of PI3K/AKT signalling, loss of PTEN, RTK overexpression, IGFR1). Targeted combinations to overcome resistance are currently being evaluated. A phase I-II study combining dabrafenib with the MEK 1/2 inhibitor trametinib (GSK1120212) showed an acceptable safety profile. Interestingly, the BRAF and MEK inhibitor combination not only demonstrated a potential reduction in drug resistance but also a lower incidence of skin toxicity [141]. Further single agent activity has been assessed in a phase-III trial, randomising patients with BRAF V600E/K mutant advanced or metastatic melanoma to receive either trametinib or chemotherapy (dacarbazine or paclitaxel). Trametinib improved rates of progression-free and overall survival [142]. In addition, many combination therapies are under evaluation and a phase I/II trial combining vemurafenib and ipilimumab was recently initiated [143]. Future strategies to overcoming the mechanisms of resistance will likely include BRAF inhibitors in combination with PI3K, PDGFR, IGF1R, or ERBB4 inhibitors.
Role of Nuclear Medicine in Melanoma Patients
Initial Staging of Primary Melanoma
Staging Nodal Metastases
Lymphoscintigraphy. In early stage melanoma (AJCC I–II), SLNB has become the standard of care for staging the regional draining lymph nodes, which are typically the first site of possible metastatic spread of disease [28, 60]. Compelling evidence from the literature indicates that SNLB is the procedure of choice for detection of occult regional lymph node metastases, particularly in patients presenting with an intermediate Breslow thickness lesion (1–4 mm) [22 23]. Preoperative lymphoscintigraphy is normally performed, followed by intra-operative detection with blue dye and/or a gamma probe [61, 62]. Precise identification of sentinel lymph node (SLN) site(s) is essential, as there may be true nodal sites, in-transit nodes and alternate drainage basins in some situations (Fig. 24.1). Lymphoscintigraphy has been shown to be highly sensitive and specific (>90%) for detection of SLN, thus assisting surgical biopsy and decisions regarding subsequent therapeutic options.
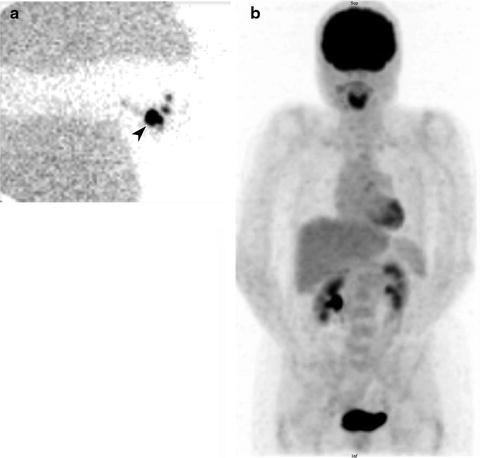

Fig. 24.1
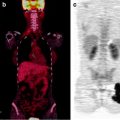
Lymphoscintigraphy of primary melanoma of the right thumb. Right axillary lymph node (arrow) identified on sentinel lymph node scintigraphy with transmission scan (a) and negative whole-body staging [18F]FDG PET scan (b)
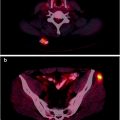
Lymphoscintigraphy for SLN detection must be performed with transmission scans, and/or with hybrid SPECT/CT systems, to precisely define the location of sentinel nodes and eliminate false-positive results. All nodes demonstrating uptake must be evaluated by detailed histology, as a SLN may not be the lymph node which demonstrates the highest tracer uptake, and different drainage basins may occur from a single primary melanoma site (Fig. 24.2). In-transit and aberrant metastatic lymph nodes occur in 14–22% of cases [61].
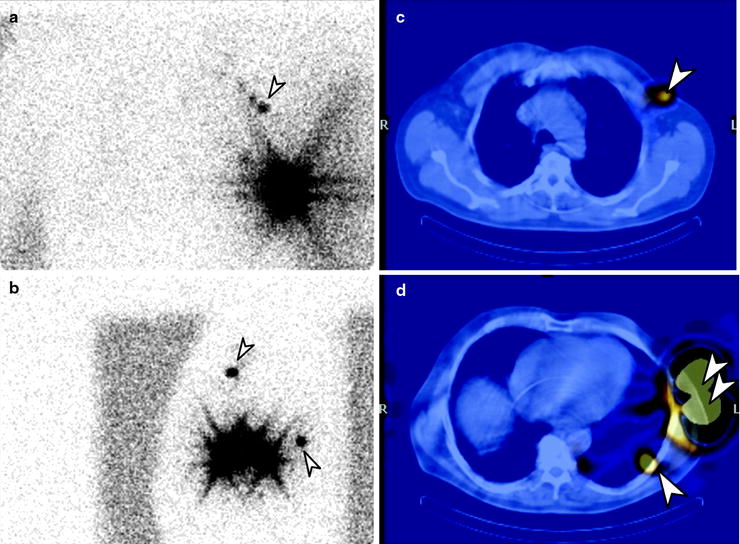

Fig. 24.2
Lymphoscintigraphy of primary melanoma of the left chest. Planar transmission scan images in the anterior (a) and lateral (b) projections. SPECT/CT confirms two separate SLN, one lateral to left pectoral muscle (c) and one in left midthoracic intercostal space (d). (Courtesy of Dr Martin Cherk, Alfred Hospital, Melbourne, Australia)
Status of the sentinel lymph node is an important prognostic factor for disease-free and overall survival. In the Multicenter Selective Lymphadenectomy Trial (MSLT-1), patients who had immediate lymph node dissection following positive SLNB had a higher survival rate than those who underwent delayed lymphadenectomy [28].
Anatomic correlation of the lymphoscintigram with SPECT/CT improves the accuracy of lymphoscintigraphy by defining anatomical site and excluding false-positive results, compared to planar gamma camera imaging. In one study of patients with primary melanoma located in the head and neck or trunk region, SPECT/CT identified “hot” nodes missed on planar images including nodes invaded by metastases in 43% of cases [62]. These SLNs identified by SPECT/CT were nodes hidden by the injection-site-scattered radiation, deeply located or in-transit nodes. Multiple draining basins were clearly identified in 50% patients with trunk melanoma and in 33% patients with head and neck malignancy. Further validation of lymphoscintigraphy with SPECT/CT in clinical studies is ongoing and may help to provide additional information on improvement in outcomes of patients evaluated preoperatively with this investigation.
[18F]FDG PET. In patients with AJCC stage I–II melanoma, multiple studies with [18F]FDG PET alone, and PET/CT, have shown a poor sensitivity for detection of metastatic disease in draining lymph nodes. Cumulative data from over 800 patients with early stage melanoma show that PET has a low sensitivity for detecting SLN metastases (Table 24.3) (60, 63, 64, 65–82). The small size of involved lymph nodes, which are often <5 mm in size, is a major factor in detection of disease, as most metastatic melanoma sites are [18F]FDG avid. In an early study by Crippa et al., the sensitivity of PET significantly varied with the size of lymph node metastases [63]. The sensitivity of PET was only 23% for lymph nodes <5 mm and increased to 83% and 100% for lymph nodes with a size of 6–10 and >10 mm, respectively. In another study exploring the impact of spatial resolution of PET scanners with typical size of metastatic lymph nodes, a spatial resolution of 4–6 mm resulted in less than 50% of lymph nodes being potentially detectable [64]. Other studies have confirmed the small size of metastatic disease in SLN and corresponding low sensitivity for PET and PET/CT for AJCC stage I–II disease [60, 65–82] (Table 24.3).
Table 24.3
Studies using [18F]FDG PET in regional lymph node staging in early-stage cutaneous melanoma
Study | Study type | Patients | AJCC stage | Clinical use of PET | Reference test | Sensitivity |
|---|---|---|---|---|---|---|
Wagner et al. [60] | Prospective | 70 | I–III | Detection of sentinel node metastases | SLNB | PET: Sensitivity 7.7% (Stage I–II), 40% (Stage III) |
Klein et al. [65] | Prospective | 17 | I–II | Detection of sentinel node metastases | LS/SLNB or clinical follow-up (20 months) | PET: Sensitivity 67% |
Acland et al. [66] | Prospective | 50 | T2–T4 or lymphatic invasion | Detection of regional lymph node metastases | LS/SLNB and clinical follow | PET: Sensitivity 0% SNB: Sensitivity 100% |
Kokoska et al. [67] | Prospective | 18 | T1–T4 N0 M0 | Detection of sentinel node metastases | LS/SLNE | PET: Sensitivity 40% |
Belhocine et al. [68] | Prospective | 21 | T2–T4 N0 M0 | Detection of sentinel node metastases | LS/SLNE | PET: Sensitivity 14% SNB: Sensitivity 86% |
Havenga et al. [70] | Prospective | 53 | T2–T4 N0 | Prediction of sentinel node metastases | LS/SLNB | PET: Sensitivity 15% SNB: Sensitivity 100% |
Longo et al. [71] | Prospective | 25 | T2–T4 N0 M0 | Prediction of regional lymph involvement | LS/SLNB | PET: Sensitivity 22% LM/LS: Sensitivity 100% |
Schafer et al. [72] | Prospective | 40 | T2–T4 N0 M0 | Detection of regional lymph node metastases | SLNB | PET: Sensitivity 0% |
Fink et al. [69] | Prospective | 48 | T2–T4 N0 M0 | Prediction of regional lymph involvement | LS/SLNB | PET: Sensitivity 13% SNB: Sensitivity 100% |
Hafner et al. [73] | Prospective | 100 | T2–T4 | Detection of regional lymph node metastases | LS/SLNB | PET: Sensitivity 8% SNB: Sensitivity 100% |
Libberecht et al. [74] | Retrospective | 5 | T2–T3 or local recurrence | Detection of regional lymph node metastases | LS/SLNB | PET: Sensitivity 0% |
Wagner et al. [75] | Prospective | 144 | T2–T4 N0 M0 or TxN2cM0 | Detection of regional lymph node metastases | LS/SLNB | PET: Sensitivity 8% SNB: Sensitivity 97.5% |
Vereecken et al. [76] | Prospective | 43 | T2–T4 or T1 with regression and/or ulceration | Preoperative staging | LS/SLNB | PET: Sensitivity 40% |
Clark et al. [77] | Retrospective | 64 | T2–T4 | Detection of regional lymph node metastases | LS/SLNB | PET: Sensitivity 10.5% |
Kell et al. [78] | Retrospective | 37 | Breslow > 0.75 mm N0 M0 | Detection of regional lymph node metastases | LS/SLNB | PET/CT: Sensitivity 22% |
Maubec et al. [79] | Prospective | 19 | T4 N0 M0 | Detection of regional lymph node metastases | LS/SLNB | PET: Sensitivity 0% SNB: Sensitivity 87.5% |
Constantinidou et al. [80] | Retrospective | 30 | Breslow > 1 mm | Preoperative staging | LS/SLNB | PET or PET/CT: Sensitivity 0% |
Singh et al. [81] | Prospective | 52 | T2–T4 N0 M0 | Detection of regional lymph node metastases | LS/SLNB | PET/CT: Sensitivity 14.3% SNB: Sensitivity 100% |
Klode et al. [82] | Retrospective | 61 | T2–T4 N0 M0 | Detection of regional lymph node metastases | LS/SLNE | PET/CT: Sensitivity 5.9% |
There is a potential role for [18F]FDG PET in patients with apparent AJCC stage I–II disease but higher risk of metastatic disease, including those with intermediate/high-risk lesions (Breslow >1 mm, regression, ulceration) or high-risk lesions (Breslow >4 mm). These patients have a prevalence of regional lymph node or distant metastases at the time of initial presentation of >10%. For example, in one study of 43 patients with intermediate/high-risk melanomas, extensive staging identified disease resulting in early treatment in 12 patients (28%) [76]. The analysis of melanomas for gene signatures that are associated with a worse prognosis is the subject of ongoing research, and this may also in the future identify patients who would benefit from more extensive staging procedures such as PET [61].
These results have led to [18F]FDG PET not being recommended for the routine detection of regional nodal involvement in AJCC I–II melanoma patients. However, PET in initial staging may have a role in situations where AJCC stage I–II patients are considered at higher risk of distant metastatic disease, such as in intermediate-high-risk or high-risk primary lesions, multiple melanomas or unusual gene signatures in melanoma lesions [83].
Staging of Metastatic Disease
Initial Staging
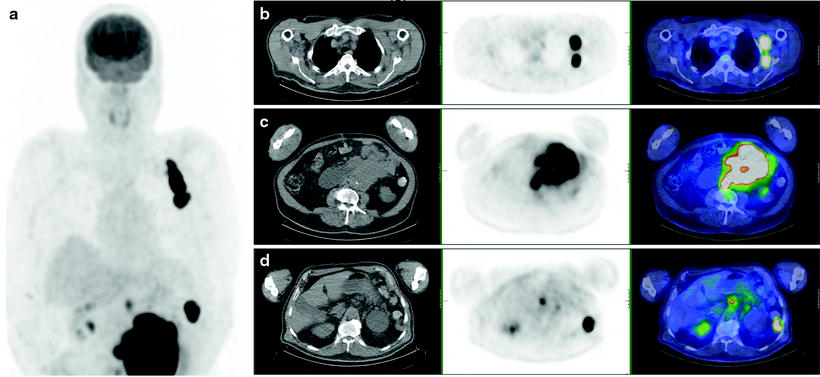
Melanoma patients presenting with AJCC stage III disease (regional nodal involvement) are at higher risk of presenting with systemic metastases, and as this can impact on therapy, accurate staging is essential (Fig. 24.3). [18F]FDG PET has been shown to be more accurate than conventional imaging techniques in identifying metastatic disease in stage III patients in a number of reported studies [84–90] (Figs. 24.4, 24.5, and 24.6). In a recent large prospective study evaluating 251 patients with PET/CT prior to surgery for known metastatic melanoma and nodal involvement, lymph node metastases were visualized in 91% of patients by PET, and 92% by CT, although in this series most lymph nodes were large [90]. Systemic metastases were suspected in 32% of patients by PET and in 24% by CT. Upstaging by PET was correct in 27% and in 24% by CT, and more bone and soft tissue sites were detected by PET compared to CT. The use of PET in assessing systemic metastatic disease in this patient group is also included in established clinical guidelines [83].
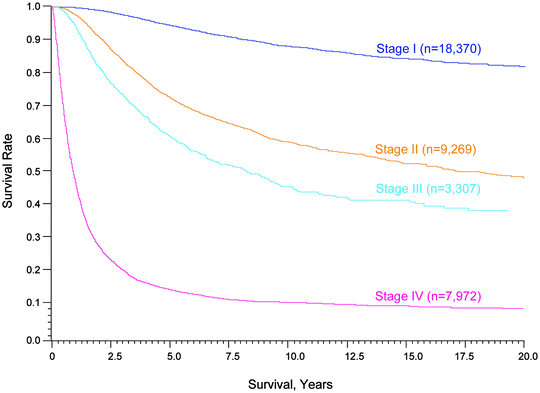
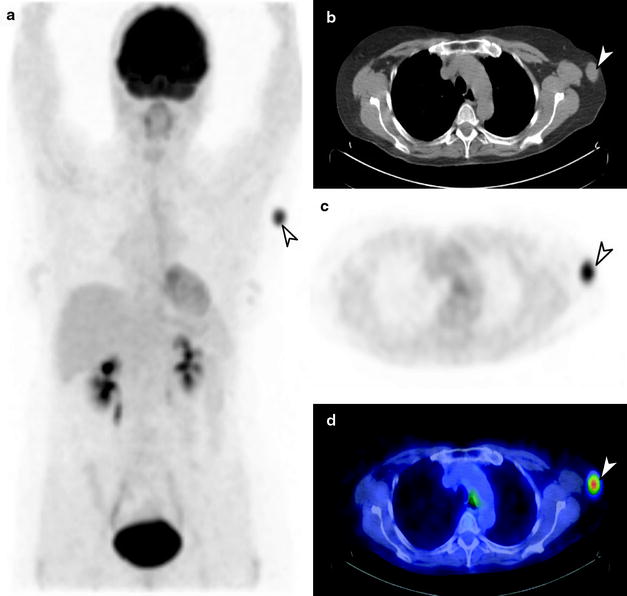
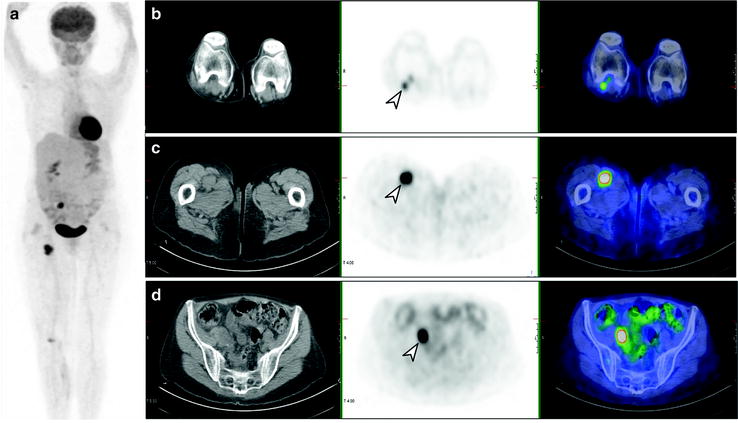
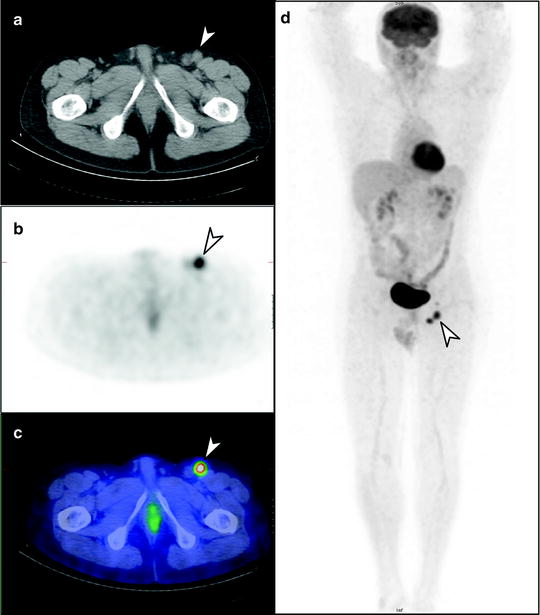

Fig. 24.3
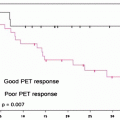
Fifteen-year survival curves for patients with localized melanoma (stages I and II), regional metastases (stage III) and distant metastases (stage IV). The numbers in parentheses are the numbers of patients from the AJCC Melanoma Staging Database used to calculate the survival rates. The differences between the curves are highly significant (p < 0.0001). (Used with the permission of the American Joint Committee on Cancer (AJCC), Chicago, IL. The original source for this material is the AJCC Cancer Staging Manual Seventh Edition (2010) published by Springer Science and Business Media LLC www.springer.com)

Fig. 24.4
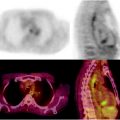
[18F]FDG PET/CT staging of biopsy-proven left axillary melanoma metastasis. Whole-body PET image (a) shows a solitary metastatic lymph node in the left axilla (arrow), with no distant metastatic disease. Also seen on axial CT (b), PET (c), and fused PET/CT (d) images

Fig. 24.5
[18F]FDG PET/CT staging post-resection of primary melanoma from right calf. Whole-body PET image (a) shows local and distant lymph node involvement in right popliteal (b), femoral (c), and iliac (d) lymph nodes

Fig. 24.6
Staging [18F]FDG PET/CT scan of left heel primary melanoma. (a) Transaxial PET, (b) CT and (c) PET/CT images show metastatic lymph nodes in the left inguinofemoral region (arrow), also seen on (d) whole-body PET image, without other metastatic disease identified
In patients with known or suspected AJCC stage IV disease (systemic metastases), [18F]FDG PET also has an important role in identifying metastatic disease sites and extent (Tables 24.4 and 24.5), which can impact on initial treatment, including surgical options and decisions regarding chemotherapy/biologic therapy [83, 91] (Fig. 24.7). The majority of melanoma metastases are [18F]FDG avid, provided lesions are >5–6 mm in size. The exceptions are brain metastases, which may be difficult to identify due to gray matter [18F]FDG uptake, and hepatic metastases of uveal melanoma, which have been shown to be negative on [18F]FDG PET in up to 59% of cases and which do not appear to be related to GLUT-1 expression [92].