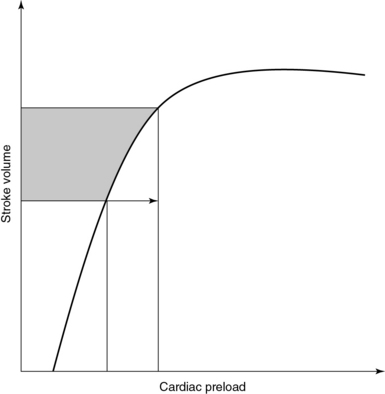
37 Prescribing fluid therapy is a common therapeutic dilemma in the intensive care unit (ICU); however, different methods of evaluating volume status are available to guide this decision. This chapter discusses these methods briefly. Fluid therapy is of critical importance in the treatment of patients in shock since it may result in improved tissue perfusion and organ function. Administration of fluids is a key feature of “goal-directed” therapy protocols in patients with septic shock inasmuch as early fluid resuscitation was suggested to improve outcomes in such patients.1 Nonetheless, overzealous resuscitation may result in tissue edema and thus impair pulmonary gas exchange, gastrointestinal motility, and wound repair. A negative impact of excessive fluid loading on outcome was demonstrated in patients with sepsis, acute respiratory distress syndrome, and renal failure.2–4 The rationale for fluid administration is the anticipated increase in cardiac output (CO) in accordance with the Frank-Starling mechanism. Starling’s law states that stroke volume (SV) increases in response to increased left ventricular end-diastolic volume or preload (Figure 37-1). Optimal preload corresponds to maximal overlap of actin-myosin fibrils. In healthy subjects, both ventricles are working on the ascending part of the Frank-Starling curve and therefore have a functional reserve in the event of acute stress.5 In critical care patients, however, the ventricles often operate on the flat part of the curve. Hence increased preload does not result in increased SV but may lead to adverse effects such as pulmonary edema. A prudent policy is to identify patients in whom CO increases in response to increased preload (fluid responsive) well before prescribing fluid therapy. Measuring volume status is rather sophisticated, whereas determining filling pressure appears to be simpler. Central venous pressure (CVP) or pulmonary artery occlusion pressure (PAOP) can be estimated by inserting a central venous and a pulmonary artery catheter, respectively. In healthy persons, CVP and PAOP should represent right and left ventricular filling pressure, respectively. Ventricular volume and pressure are linked by the volume-pressure curve. Increments in end-diastolic volume result in increased end-diastolic filling pressure. Unfortunately, there is no linear correlation between volume and pressure. Recently, it was demonstrated that both CVP and PAOP failed to predict changes in end-diastolic ventricular volume after the infusion of 3 L of saline into healthy volunteers.6 If this principle does not apply to healthy subjects, it may indeed be of limited value in the ICU. Remarkable changes in ventricular compliance and intrathoracic pressure take place in the critically ill, mainly because most of them are mechanically ventilated and under the influence of vasoactive agents (e.g., inotropes). The impact of these changes on determination of CVP or PAOP is unpredictable. Surely, CVP is not associated with circulating blood volume and does not predict fluid responsiveness.7 Accordingly, determination of PAOP is not recommended as a predictor of fluid responsiveness. Despite the aforementioned considerations, CVP and PAOP are routinely used as measures of volume status in the ICU. Surveys have confirmed that more than 90% of intensivists use CVP to guide fluid therapy.8 The Surviving Sepsis Campaign recommended that septic patients be fluid-resuscitated to a CVP goal of 8 to 15 mm Hg.9 This might be due to the fact that central venous catheters are standard tools in the hands of intensivists. Also, it is not always easy to alter clinical notions that have been shaped in a particular manner over a long period. If CVP is used to guide fluid therapy, single point estimations should not be interpreted in isolation but always in the context of pertinent clinical scenarios. Measuring end-diastolic filling volume is challenging, although estimates can be obtained with the transpulmonary thermodilution method. The latter is integrated into the PiCCO system (Pulsion Medical Systems AG, Munich, Germany). After injecting a cold saline bolus via a central line, the temperature is recorded with a large arterial thermistor. Mathematical analysis of the thermodilution curve provides the global end-diastolic volume (GEDV). This virtual volume reflects the volume of all four cardiac chambers in diastole. Several studies have demonstrated that GEDV is superior to filling pressure in estimating fluid responsiveness in various clinical scenarios.10 The main issue is defining normal ranges of GEDV even after it is indexed for body surface area; moreover, GEDV seems to be influenced by age, gender, and left ventricular function.11,12 Thus application of GEDV measurements in an individual patient may be difficult to interpret.
Measures of volume status in the intensive care unit
Overview
Pressure-related techniques
Static volume-based parameters
Radiology Key
Fastest Radiology Insight Engine