Chapter 36 Melanoma
Introduction
The utility of imaging studies in patients with melanoma generally depends on the melanoma stage. There is little, if any, role for comprehensive imaging in patients with early-stage disease where surgery is often curative.1 Preoperative lymphoscintigraphy is generally first performed to identify site(s) of regional nodal drainage, including those patients with multiple draining nodal basins as well as to locate unpredictable nodal basins outside the standard nodal basins. Intraoperative lymphatic mapping and sentinel lymph node biopsy provide accurate staging of melanoma patients with no clinically detectable nodal disease.2 Hematogenous dissemination is more likely in advanced regional disease and can occur in any organ system and, unfortunately, at any time. For distant metastases, computed tomography (CT) and magnetic resonance imaging (MRI) are the dominant imaging modalities in general use. Ultrasound is commonly performed to evaluate surgical scars, new palpable lesions, lymph nodes with equivocal findings on other examinations, and/or lymph node basins at risk for metastatic disease. Positron-emission tomography (PET)/CT has an extremely limited, if any, role in patients with early disease (stages I and II) with no current universally accepted role for routine use.3 It can be used in patients with potentially resectable stage IV disease to exclude other sites of disease and for problem-solving for equivocal findings on other imaging studies. Imaging, therefore, plays an important role in staging, treatment planning, and posttreatment follow-up of patients with melanoma.
Epidemiology and Risk Factors
Epidemiology
Melanoma accounts for 4% to 5% of new cancer diagnoses in the United States and is the fifth and sixth most common new cancer diagnoses in men and women, respectively.4 It is estimated that there will have been 70,230 new cases of melanoma diagnosed, and 8790 deaths from melanoma in 2011.4 The lifetime risk for developing melanoma is approximately 1 in 50.5 Furthermore, its incidence continues to increase throughout much of the world. Although the exact etiology for these epidemiologic trends is not entirely clear and certainly multifactorial, the rising incidence is thought to be at least partly related to increased sun exposure and early detection through screening programs.
The incidence of melanoma also has a notable geographic variation, with the highest incidence in Australia/New Zealand, followed by Europe and North America, respectively. Whereas the reasons for this are multiple and likely multifactorial, it is likely at least in part due to the sun exposure and fair skin complexions of populations in these countries.6
Risk Factors
In men, approximately one third of melanomas are on the trunk, most commonly the back, and in women, the most common site is in the lower extremities (42%).7 These distributional differences may reflect sun exposure patterns between the sexes.
Melanomas are generally associated with nevi, particularly dysplastic nevi. A family history of melanoma is also associated with an increased risk, possibly because of mutations in CDKN2A and CDK4 genes. A variety of susceptibility genes have also been identified.7
Anatomy and Pathology
Anatomy
Melanomas are derived from melanocytes, the pigment-producing cells in skin, and are of neural crest origin. These cells are distributed widely throughout the skin as a result of their embryologic origin. Melanocytes can also be found in other sites and give rise to uncommon primary melanomas. Examples of noncutaneous sites in which melanomas occur include the eye (e.g., conjunctiva, retina, and uveal tract) and mucosae (e.g., anal, buccal, and nasal).8 Primary visceral melanoma is extremely rare but has also been reported.9–11
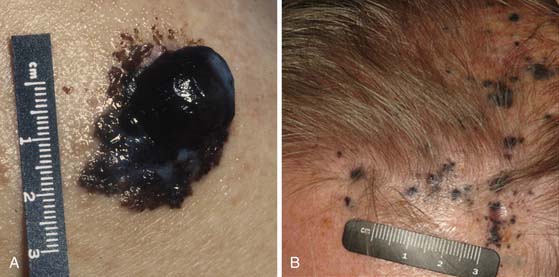
Melanomas typically contain melanin, which accounts for their characteristic dark appearance (Figure 36-1). However, some melanomas do not contain obvious visible melanin and are termed amelanotic.
Pathology
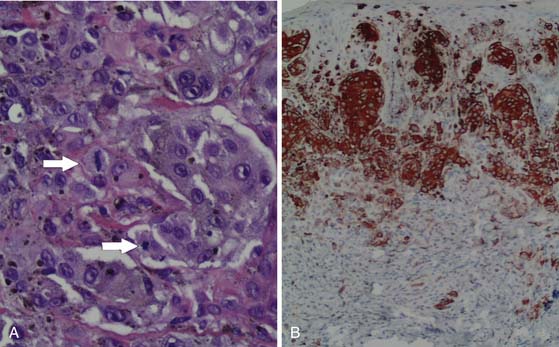
Although the appearance of some cutaneous lesions is highly suggestive of melanoma, definitive diagnosis is based on histologic assessment of the primary lesion. In general, the diagnosis of melanoma is based on a combination of standard hematoxylin and eosin (H&E) staining as well as confirmatory immunohistochemical studies using, for example, antibodies against melanocytic antigens such as S100 protein, melan-A, or gp100 (as detected by the antibody HMB45) (Figure 36-2). HMB45 can help distinguish melanoma in situ from sun-damaged skin/pigmented actinic keratosis. A confluent pattern of growth is observed with melanoma in situ compared with scattered atypical melanocytes found in sun-damaged skin or a pigmented actinic keratosis.12
Cutaneous melanomas typically grow from the epidermis toward the subcutaneous tissues (through papillary dermis, then reticular dermis, then subcutaneous tissues). The risk of metastatic disease and the prognosis of cutaneous melanoma are associated with the thickness of the primary lesion at the time of diagnosis, the presence or absence of primary tumor ulceration, and mitotic rate; hence, the need for careful histologic evaluation of the primary melanoma is paramount. The degree of proliferation of melanocytes is an important prognostic marker, for which the proliferation marker Ki-67 may also be employed.12
Key Points Anatomy and pathology
• Melanomas are derived from melanocytes, originating from the neural crest.
• Melanomas typically contain melanin, but some do not contain significant amounts of melanin and are classified as amelanotic.
• Melanomas most commonly arise in the skin and may arise in other areas (e.g., eye and mucosae).
• Diagnosis must be confirmed histologically.
• Tumor thickness is a key prognostic marker in cutaneous melanoma.
• Other primary tumor prognostic markers include ulceration and mitotic rate.
Clinical Presentation
The vast majority of patients with cutaneous melanoma present with a new or changing skin lesion that can occur essentially anywhere on the body. These are typically, but not always, darkly pigmented; pigmentation may be heterogeneous or homogeneous. Lesions may or may not be raised and nodular (see Figure 36-1). The diagnosis of cutaneous melanoma on clinical examination is fraught with difficulty and error; a reliable diagnosis of melanoma requires biopsy and histopathologic evaluation. Biopsies should be strongly considered for any pigmented lesions that have a change in color, size, or morphology. The ABCDEs of lesion classification as described by Rigel and coworkers13 may be used to identify skin lesions suspicious for melanoma and deserving of biopsy. “A” describes asymmetry, “B” describes border irregularity, “C” is for color variegation, “D” denotes a diameter greater than 6 mm, and “E” is for a lesion that is evolving or enlarging. A small subset of skin lesions ultimately confirmed to be melanoma that are not hyperpigmented are classified as amelanotic. In some patients with a newly diagnosed primary melanoma, a synchronous or second primary can be found.
In-transit and satellite metastases, defined as cutaneous or subcutaneous metastatic deposits between the primary tumor and the regional nodes may occur, represent a clinically relevant form of lymphatic metastasis in patients with melanoma. This pattern of recurrence is found in 3% to 12% of patients with melanoma.14
Patterns of Tumor Spread
Lymphoscintigraphy is used for localization of sentinel lymph nodes before surgery. The sentinel lymph node is the first draining lymph node on the afferent lymphatic pathway from the primary tumor. However, more than one pathway (i.e., more than one sentinel node and/or regional nodal basin) may drain a primary tumor. Nodes that are found along the course of a lymphatic pathway between the primary melanoma and the recognized regional nodal field(s) are termed interval (in-transit) nodes.15 Uren and colleagues15 found that interval nodes were more common in truncal melanomas than in those found in the lower limbs. Recognition of interval nodes is clinically important; they along with sentinel nodes in defined regional nodal basins should be removed during surgery because the interval node may be the only node containing metastatic disease. Thompson and associates16 found 13 of 4262 patients (0.31%) with primary melanomas of the distal lower extremities to develop popliteal nodal metastases. Because popliteal nodal involvement from melanoma is uncommon, indications for popliteal nodal dissection include a positive histologic popliteal fossa sentinel node or clinical detection of popliteal nodal disease.16 Uren and coworkers17 observed the drainage pathway to the epitrochlear region was seen in 36 of 218 patients (16%) with melanomas on the forearm and hand.
A feature of lymphatic dissemination in cutaneous melanoma, which is not commonly seen in other tumors, is “in-transit” disease. In-transit disease is the presence of cutaneous or subcutaneous melanoma deposits located between the primary tumor and the regional draining nodal basin. Risk factors for in-transit disease include age older than 50, a lower extremity primary tumor, increasing Breslow depth, ulceration, and positive sentinel lymph node status.14,18–21
Primary uveal melanoma, unlike primary cutaneous melanoma, tends to preferentially metastasize hematogeneously (rather than via lymphatics), most commonly to the liver. In patients with metastatic uveal melanoma, the liver is involved in 71.4% to 87% of patients. Liver metastases can appear up to 15 years after the initial diagnosis of the primary uveal melanoma.22–25 Uveal melanoma cells have been shown in studies to express receptors (e.g., c-Met, insulin-like growth factor-1 receptor and CXCR4) for ligands (e.g., hepatocyte growth factor, insulin-like growth-factor-1 and stromal-derived factor-1) produced in the liver. The binding of these receptors and ligands contributes to cell growth and motility and increased invasiveness.26
Key Points Tumor spread
• Lymphatic dissemination typically precedes hematogenous spread, most commonly to regional nodes.
• Satellite and in-transit metastases—defined as cutaneous or subcutaneous metastatic deposits between the primary tumor and the regional nodes—may occur and represent a clinically relevant form of lymphatic metastasis in patients with melanoma.
• Risk factors for in-transit metastases include age older than 50, lower extremity primary tumor, increasing Breslow depth, ulceration, and positive sentinel lymph node status.
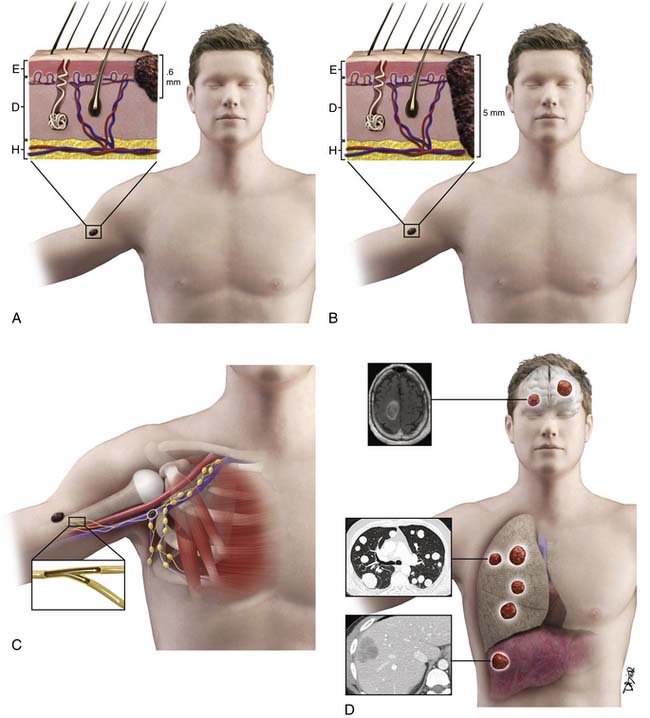
• Hematogenous metastases may occur anywhere, although “common” sites of distant metastatic melanoma include lung, soft tissue, liver, and brain.
Staging and Prognosis
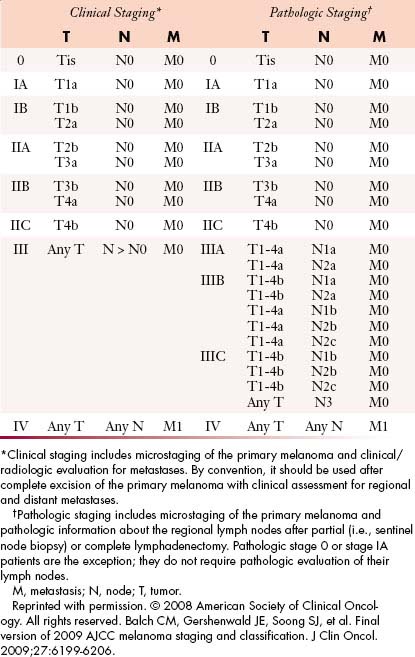
The TNM (tumor-node-metastases) classification is used for staging melanoma. The sixth edition of the American Joint Committee on Cancer (AJCC) staging for cutaneous melanoma published in 2002 has been superseded by the seventh edition of the AJCC Cancer Staging Manual, published in 2009 and implemented in January 2010.27 Assessment of the primary tumor and the presence or absence of regional nodal and distant metastases are the basis for staging (Tables 36-1 and 36-2 and Figure 36-3).
Table 36-1 Tumor-Node-Metastasis Staging Categories for Cutaneous Melanoma
| CLASSIFICATION | THICKNESS (mm) | ULCERATION STATUS/MITOSES |
|---|---|---|
| T | ||
| Tis | NA | NA |
| T1 | ≤1.00 | a Without ulceration and mitosis < 1/mm2 |
| b With ulceration or mitoses ≥ 1/mm2 | ||
| T2 | 1.01-2.00 | a Without ulceration |
| b With ulceration | ||
| T3 | 2.01-4.00 | a Without ulceration |
| bWith ulceration | ||
| T4 | >4.00 | a Without ulceration |
| b With ulceration | ||
| NO. OF METASTATIC NODES | NODAL METASTATIC BURDEN | |
| N | ||
| N0 | 0 | NA |
| N1 | 1 | a Micrometastasis* |
| b Macrometastasis† | ||
| N2 | 2-3 | a Micrometastasis* |
| b Macrometastasis† | ||
| c In-transit metastases/satellites | ||
| without metastatic nodes | ||
| N3 | 4+ metastatic nodes, or matted nodes, or in-transit metastases/satellites with metastatic nodes | |
| SITE | SERUM LDH | |
| M | ||
| M0 | No distant metastases | NA |
| M1a | Distant skin, subcutaneous, or nodal metastases | Normal |
| M1b | Lung metastases | Normal |
| M1c | All other visceral metastases | Normal |
| Any distant metastasis | Elevated | |
M, metastasis; N, node; NA, not applicable; T, tumor.
* Micrometastases are diagnosed after sentinel lymph node biopsy.
† Macrometastases are defined as clinically detectable nodal metastases confirmed pathologically.
Reprinted with permission. © 2008 American Society of Clinical Oncology. All rights reserved. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
Tumor
The T category is based on primary tumor thickness, determined by measuring the maximal thickness from the top of the epidermal granular layer to the bottom of the tumor, using an ocular micrometer.28 Strata are primarily defined as T1 to T4. T1 lesions are melanomas that are 1.0 mm or less in thickness (and are also known as thin melanomas). A T2 lesion is 1.01 to 2.0 mm and a T3 lesion is 2.01 to 4.0 mm (together they compose intermediate-thickness melanoma). Melanomas greater than 4.0 mm are T4 lesions and are clinically classified as a thick melanoma. In addition to these strata, a Tx lesion is a primary tumor that cannot be assessed, such as a regressed melanoma or one associated with a curettage biopsy. T0 indicates no evidence of primary tumor and a Tis lesion indicates melanoma in situ (i.e., noninvasive melanoma). The T category is further subcategorized according to the presence or absence of primary tumor ulceration and, for T1 lesions only, the presence or absence of mitotic activity (histologically defined as mitoses/mm2).27 Primary tumor thickness at the time of diagnosis is the major determinant of prognosis and is used for treatment planning. For ulcerated lesions, primary tumor thickness is measured from the ulcer base to the bottom of the tumor. The level of invasion, originally defined by Clark, is no longer in use based on its limited independent prognostic significance in contemporary multivariate modeling.27 Instead, tumor thickness, tumor ulceration, and mitotic rate are the most powerful primary tumor prognostic factors in patients with primary melanoma. There is an inverse relationship between tumor thickness and prognosis with a significant decrease in survival with increasing tumor thickness. The same holds true for mitotic rate. Patients with an ulcerated melanoma have a lower survival rate than those patients with a nonulcerated primary lesion.27
Node
The primary criterion for the N category is defined by the number of metastatic lymph nodes present. The N category is divided into N1 to N3. The N1 category includes patients with one metastatic node. Those patients with two or three metastatic nodes are classified as N2, and those with four or more metastatic nodes or matted nodes are considered N3.
Tumor burden, the second most important prognostic factor in patients with nodal metastases, thus far has been empirically subdivided into micrometastases and macrometastases. Patients without clinical or radiologic evidence of lymph node metastases, whose regional disease was identified by sentinel node biopsy or, previously, elective lymph node dissection, are considered to have micrometastases; in contrast, those patients with clinical evidence of metastatic disease (pathologically confirmed) are classified to have macrometastases. The third and final criterion of the N category includes the presence or absence of satellites or in-transit metastases.27
Stage Groupings and Prognosis
According to the AJCC staging system, stages I and II groupings are primary melanomas without evidence of nodal or distant metastases. Stage III groupings include nodal involvement, and stage IV includes nodal and distant metastases. Current stage groupings are summarized in Table 36-2.
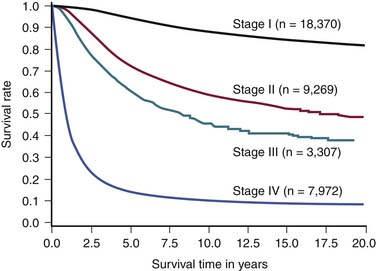
Among all patients with cutaneous melanoma, the prognosis is rather heterogenous. The 2009 AJCC survival rates for patients with cutaneous melanoma by stage are shown in Figure 36-4.27 For example, the 5-year survival rate is over 98% in patients with a T1a (i.e., thin) primary melanoma.27 In patients with regional lymph node metastases (i.e., stage III) the prognosis is highly variable. For example, in patients with nonulcerated primary tumors with only a single microscopically involved regional lymph node, the 5-year survival rate is approximately 80%, whereas the 5-year survival for patients with four or more clinically involved lymph nodes decreases to 25%. Overall, patients with distant metastatic melanoma (i.e., stage IV) have a poor prognosis. Patients in the AJCC M1a category have a 1-year survival rate of 62%. Patients in the AJCC category M1b have lung metastases with a 1-year survival rate of 53%. This decreases to a 33% 1-year survival rate in patients with distant metastases and an elevated serum LDH (M1c).
Key Points Staging
• Primary tumor thickness, presence of ulceration, and mitotic rate are the dominant independent predictors of survival.
• The risk of metastatic disease increases with T stage.
• Level of invasion is no longer used as a primary tumor criterion for AJCC staging.
• In patients with negative regional lymph nodes on clinical examination, intraoperative lymphatic mapping and sentinel lymph node biopsy are used to surgically stage regional nodal basins at risk
• The primary criterion for the N category is defined by the number of metastatic regional lymph nodes. Most patients who present with regional nodal disease have evidence of microscopic nodal disease identified by lymphatic mapping and sentinel node biopsy.
• Patients with distant metastasis are classified by site(s) of metastases (M1a, M1b, and M1c) and LDH level. Patients with an elevated LDH level have a significant reduction in survival.
Imaging
Primary Tumor
The T staging of cutaneous melanoma is based on histologic evaluation of the primary tumor. In general, there is no role of imaging in T staging of cutaneous melanoma. In rare cases, high-frequency ultrasound can be used to assess large primary tumors for which complete excision prior to treatment is not feasible, particularly when it may impact on margins of excision. Hayashi and colleagues29 studied melanomas with high-frequency (30-MHz) ultrasound. In 68 of 70 sonographically well-seen melanomas (excluding two melanoma in situ lesions), they showed good correlation between sonographic and histologic thickness (r = 0.887) and found high-frequency sonography to be quite useful in preoperative prediction of tumor thickness.29