CHAPTER 30 Meningeal Neoplasms
The meningeal coverings of the human brain are composed of three distinct anatomic structures, each of which has two component layers.
The arachnoid, which coats the inner surface of the dura, is also composed of two layers: the more superficial or arachnoid cap layer and the deeper arachnoid trabecular layer. These layers vary in thickness in different regions of the brain. The pia mater, which is innermost and immediately covers the brain, is composed of two layers: the epipia and the intima pia. Between the arachnoid and the pia is the subarachnoid space, which is filled with cerebrospinal fluid and is variable in size, enlarging to form the subarachnoid cisternal spaces at the base of the brain and narrowing over the cerebral convexities.1
The embryology of the meninges is a subject of ongoing study, with some believing that the dura mater is of mesodermal origin and that the leptomeninges are of ectodermal origin.2 Comparative studies in birds show that the leptomeninges are at least partially of neural crest origin.3,4 In any case, meningeal tissues and the neoplasms they give rise to are capable of exhibiting a wide range of mesodermal, ectodermal, and even neuroectodermal characteristics.
• Meningothelial neoplasms or meningiomas
• Nonmeningothelial mesenchymal neoplasms, both benign and sarcomatous
• Glial tumors of the leptomeninges
• Tumors of hematopoietic origin
• Miscellaneous neoplastic and non-neoplastic masses and cysts
MENINGIOMAS
In 1922, Harvey Cushing introduced the term meningioma to refer to a disparate group of intracranial, extra-axial neoplasms, which had been previously referred to by many different names, most commonly dural endotheliomata. Sixteen years later, Dr. Cushing and Dr. Louise Eisenhardt solidified the diagnostic nomenclature with their monograph entitled Meningiomas. They described their experience with 313 tumors, codifying 22 different histologic subtypes of this tumor, and providing convincing clinical and pathologic evidence that these different tumors were all variants of a single entity.5
Despite subsequent advances, particularly in the fields of molecular biology, much of their work has withstood the test of time. The generally accepted cell of origin of meningioma is the arachnoid cap cell, which is found in the most superficial layer of the arachnoid tissues. The arachnoid origin was first suggested by Bright in 1831,5 then more conclusively demonstrated by Cleland in 1864.5 In 1902, Schmidt5 pursued a more detailed dissection, allowing the observation that these tumors arose from the most superficial layer of the arachnoid or, according to current terminology, the arachnoid cap cells. Arachnoid cap cells are most commonly found on the surface of the brain in association with dural sinuses and dural reflections but are also present in the tela choroidea, which extends throughout the ventricular system of the cerebrum. The arachnoid component of the tela choroidea gives rise to the less common intraventricular meningioma. Rarely, meningiomas are found elsewhere in the body, including intraosseous, intracerebral nondural,6 and pulmonary locations.7 The histogenesis of these tumors is uncertain; however, it is hypothesized that these distant or ectopic meningiomas may arise from arachnoid tissue associated with nerve sheaths or from ectopic meningothelial cells or possibly from pluripotential stem cells.
Epidemiology
Meningiomas are one of the most common intracranial neoplasms, representing approximately 25% of all new brain tumor diagnoses per year.8 There is roughly a 1.5 to 2.0 female-to-male ratio, and the peak age at diagnosis is 60 to 70 for men and 70 to 80 for women. Interestingly, the female preponderance is not present in atypical and pediatric meningiomas but is increased to 8 to 10:1 in spinal meningiomas. Female preponderance is also lacking in Africans and African Americans, who, as a group, have a slightly higher incidence of meningiomas than persons of either European or Asian descent.9,10 Incidence values vary in different studies, but a large meta-analysis gives the figure of 2.6 per 100,000 overall, and this is increased to 3.1 per 100,000 in those of African heritage.11
Meningiomas may be subdivided into many different categories according to histology, location, degree of biologic aggressivity, morphology, and demographic features. The classification scheme that has the most clinical relevance in terms of treatment, prognosis, and natural history is that adopted by the World Health Organization (WHO) in its 2007 revision of brain tumor taxonomy,12 which divides meningiomas into three categories: benign, atypical, and malignant or anaplastic.
Benign—WHO grade I: 80% to 90%
• 1. Histologic atypia is lacking (e.g., increased mitotic index)
• 2. Certain specific histologic subtypes include meningothelial, fibroblastic, transitional, psammomatous, microcystic, lymphoplasmacytic, angiomatous, secretory, and metaplastic
• 3. Benign typical meningiomas may, and frequently do, exhibit invasion of adjacent soft tissues and bone, but brain invasion is indicative of at least grade II
Atypical—WHO grade II: 8% to 10%
• 1. Mitoses greater than 4 per 10 high-power fields
• 2. Certain histologic subtypes: clear cell and chordoid
• 3. Brain invasion. There has been an evolution of opinion regarding brain invasion. In the absence of frank anaplasia, meningiomas that exhibit brain invasion have been shown to be prognostically similar to atypical grade II, rather than malignant grade III, tumors. In the previous WHO classification, brain invasion alone connoted malignant or grade III, whereas in the latest edition it merits a grade II designation.
• 4. Three of five specific histologic findings, including spontaneous necrosis, hypercellularity, sheeting architecture, small cell formation with a high nuclear to cytoplasmic ratio, and/or macronucleoli
Malignant—WHO grade III: 1% to 2%
• 2. Certain histologic subtypes: rhabdoid, and papillary
• 2. Mitotic index greater than 20 per 10 high-power fields, necrosis, and direct brain invasion
The age of the patient is also important. Some data suggest that meningiomas arising in younger individuals are more aggressive, although definitive statistical evidence has not been collected.13–15
Etiology
The etiology of meningiomas is multifactorial. There is clear evidence that local irradiation is associated with an increased incidence of meningiomas. Head trauma was believed by Cushing to be causative; however, this has not been confirmed and remains controversial.16
Viral particles of many types are found within the genetic material of meningiomas, which provides circumstantial evidence for an etiologic role in their growth. For example, papovaviruses such as BK and SV40, as well as adenovirus DNA, have been found in meningiomas. The exact role, if any, that viral genetic material plays in meningioma origin and/or growth remains uncertain.17
Pathophysiology
The genetic basis for meningiomas is currently under intensive ongoing study.18 As in many other neoplasms, meningiomas are believed to arise by a multi-hit mechanism of genetic damage or abnormality, possibly involving damage or absence of a suppressor gene, as well as amplification or abnormality of an active pro-oncogene.19 The most common genetic abnormality associated with meningioma is that found on chromosome 22q12, which is implicated in neurofibromatosis type 2 (NF2). Meningiomas are part of the NF2 syndrome, which is associated with multiple inherited schwannomas, meningiomas, and ependymomas, giving rise to the mnemonic MISME, pronounced “miss me.” NF2 is associated with abnormal gene function at chromosome 22q12, which codes for a protein named merlin or schwannomin. Merlin’s function is still under study, but it is believed to regulate cell-cell contact inhibition and also to play a role in cell morphology. Inactivation would therefore result in cells with altered morphology and impaired contact inhibition. NF2 mutations are seen in patients with NF2 and also in more than half of patients with sporadic meningiomas. It has also been shown that other chromosome 22 mutations distinct from the NF2 locus are common in meningiomas, suggesting that there may be another tumor suppressor locus on chromosome 22 distinct from that which encodes for merlin.20,21 Some data suggest that different subpopulations of meningiomas based both on histology (transitional and fibrous) and location (anterior skull base) may be more likely to show merlin inactivation than others. Meningothelial tumors only rarely show reduced expression of merlin, and radiation-induced meningiomas also are associated with decreased likelihood of chromosome 22q12 abnormalities but are more likely to display structural abnormalities of chromosome 1p.
Chromosomal and genetic abnormalities that have been found in patients with meningiomas and particularly in patients with higher grade II and grade III meningiomas include dicentric or ring chromosomes, absence or alterations in 1p, 3p, 6q, 9p, 10q,22 and 14q23, and amplifications in 17q and 20q. These abnormalities may be associated both with origination of the meningothelial neoplasm and possibly also progression in grade.
Other specific genetic changes associated with atypical (grade II) and malignant or anaplastic (grade III) meningiomas include expression of the enzyme telomerase, which allows cells to maintain telomere length over multiple cell division cycles.24 Proteins associated with increased angiogenesis, including tenascin and vascular endothelial growth factor, are also found in higher-grade meningiomas. In one group, 25% of malignant meningiomas showed deletions of the CDKN2A locus on chromosome 9p21. Other specific findings include the loss of the NDRG2 gene on chromosome 14q11.2 and the gain of the RPS6KB1 gene on chromosome 17q23 (Fig. 30-1).
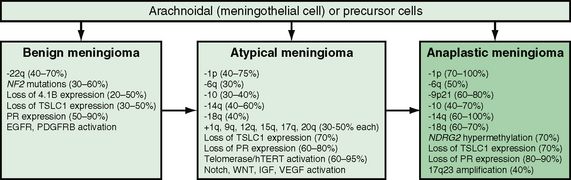
FIGURE 30-1 Genetic model of meningioma tumorigenesis and malignant progression. EGFR, Epidermal growth factor receptor; hTERT, telomerase reverse transcriptase; IGF, insulin-like growth factor; NDRG2, N-Myc downstream regulated gene 2; PDGFRB, platelet-derived growth factor receptor, beta polypeptide; PR, progesterone receptor; TSLC1, tumor suppressor in lung cancer-1; VEGF, vascular endothelial growth factor.
(From Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO Classification of Tumors of the Central Nervous System. Albany, NY, World Health Organization Publications Center, 2007.)
Meningiomas express a wide variety of cell surface proteins and receptors, including hormonal receptors.25 Vimentin is virtually always present, and epithelial membrane antigen (EMA) reactivity is seen in the majority of cases. EMA is somewhat more specific than vimentin, which is generally diffusely positive but nonspecific. Claudin-1 may be useful in addition to EMA for ambiguous cases.
The role of hormonal receptors in meningioma growth is complex and not fully understood. Estrogen, progesterone, and androgen receptors may be found in meningiomas. Immunopositivity for progesterone is by far more common than other hormone receptors.26 Progesterone receptors have been shown to stimulate meningeal cell growth in vitro culture, and progesterone receptor inhibitors have been shown to have the opposite effect. In addition, the extent of progesterone receptor expression appears to be inversely proportional to tumor grade and proliferation.27 Accordingly, benign tumors are more likely to express immunoreactivity to progesterone receptor antigens (50% to 80%). Estrogen receptors are somewhat less commonly expressed than progesterone receptors. The relationship between gender and hormonal receptor status remains an area of ongoing study.28
Imaging
Radiography
Meningiomas exhibit several imaging features that may be seen on plain radiographs. Meningiomas may stimulate hyperostosis in underlying bone, which is believed secondary to secretion of factors that stimulate osteoblasts. This is also observed on nuclear medicine bone scintiscans as increased radiotracer deposition. As meningiomas grow through the calvaria, they may produce an exophytic hyperostosis in the scalp referred to as “hair-on-end” appearance (Fig. 30-2). Finally, meningiomas may contain psammomatous regions, and if dense enough these may be seen as hazy areas of calcification.
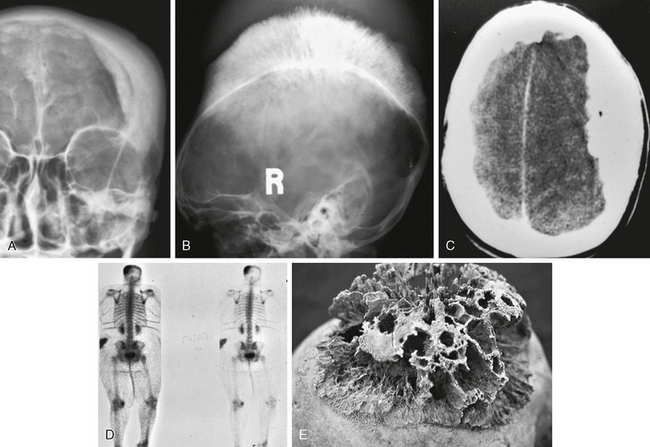
FIGURE 30-2 Osseous manifestations of meningiomas in different patients. Frontal and lateral skull radiographs (A, B), and axial CT image (C) show prominent hyperostosis with typical hair-on-end appearance. Bone scintiscan (D) shows increased radiotracer uptake. Postmortem skull (E) shows marked, coarse, hyperostotic changes.
Angiography
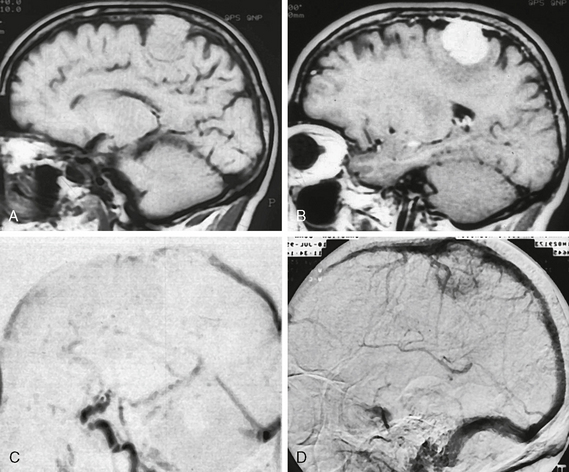
Although less commonly used today, angiography was once the mainstay of diagnostic evaluation of intracranial tumors. Meningiomas are distinctive owing to their primary arterial supply from the middle meningeal branch of the external carotid artery or secondarily by posterior meningeal artery branches (Fig. 30-3). They also typically show early brisk arterial filling and delayed venous washout, sometimes referred to as the “the in-law sign” because of the pattern of arriving early and staying late. Angiographic visualization of meningiomas is currently performed primarily in order to perform preoperative embolization, which has been shown to decrease blood loss in difficult cases. Angiographic venous phase or MR venographic images may also be important to document impingement on dural sinuses (Fig. 30-4).
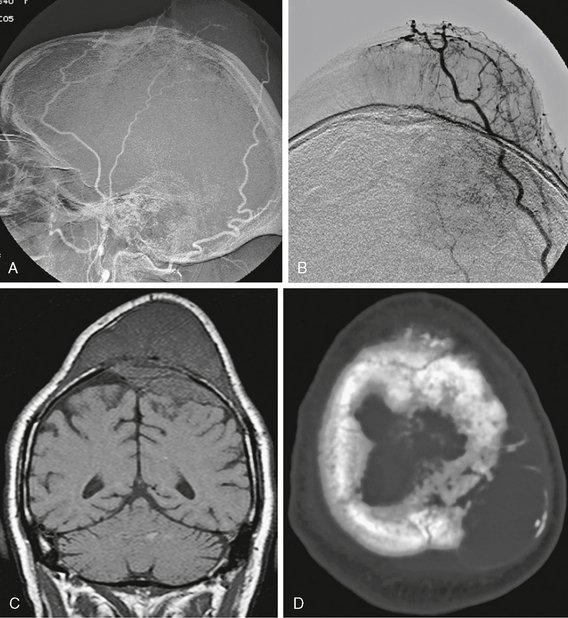
FIGURE 30-3 Angiographic images (A, B) show typical middle and posterior meningeal artery supply to this large transcalvarial meningioma. Coronal T1W MR image (C) shows the substantial intracalvarial and extracalvarial soft tissue components of this mass and axial CT scan (D) shows the intact but altered bony structure at the tumor site.
CT
On CT, meningiomas are generally hyperdense to brain parenchyma and enhance briskly and uniformly. If histologic variants are present, different imaging appearances may predominate. Microcystic meningioma may exhibit low density nearer to water. If lipomatous metaplasia has occurred, fatty density is seen (Fig. 30-5). Psammomatous calcifications may be present, giving a hazy sand-like appearance, whereas coarse calcifications may be observed with osseous metaplasia. Hyperostosis generally reflects thickening of bone adjacent to the tumor. Perilesional edema may be seen as low density following white matter tracts. Some meningiomas, presumably after many years, may become densely calcified throughout.
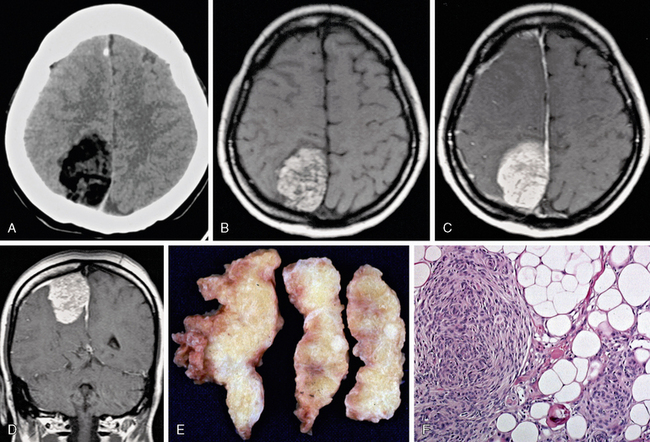
FIGURE 30-5 Lipomeningioma. Low density is seen on noncontrast CT (A). Fatty high T1 signal intensity is seen on a nonenhanced T1W image (B), which is somewhat less obvious on postgadolinium axial and coronal T1W MR images (C, D). Gross pathologic specimen (E) shows typical yellow fatty tissue, and hematoxylin and eosin–stained (×100) microscopic specimen (F) shows lipid globules.
MRI
The great majority (80% to 90%) of meningiomas are grade I, and this figure may be higher if small asymptomatic tumors are included. The majority of these tumors display typical meningothelial histology without metaplastic or other atypical features. Typical imaging features consist of a rounded, smooth and well-circumscribed extra-axial mass that is broad based against the dural surface. These lesions show dense and homogeneous enhancement, frequently with adjacent dural thickening and enhancement described as the “dural tail” (Fig. 30-6). This dural tail has been found to be reactive in most cases and may not represent extension of the neoplasm. It is suggestive but not specific for meningioma, and it is commonly seen in other dural-based tumors as well.29,30 Most common locations include the frontal convexities and the falx as well as the sphenoid wing. Other typical but less common locations include the orbital sheath, the tentorium cerebelli, the petroclinoid ligaments, the cavernous sinuses, and the olfactory grooves (Fig. 30-7).
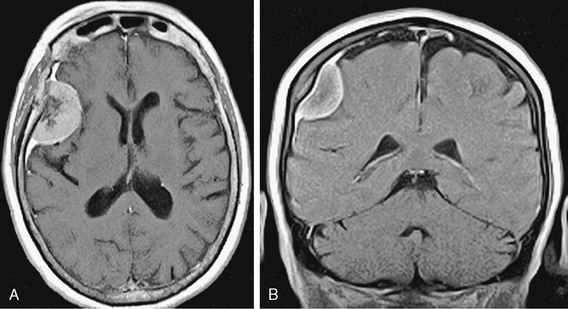
FIGURE 30-6 Axial (A) and coronal (B) postgadolinium T1W MR images show dense homogeneous enhancement and a “dural tail.” Some hyperostosis is also present. The dark signal intensity centrally is due to psammomatous or sand-like calcifications.
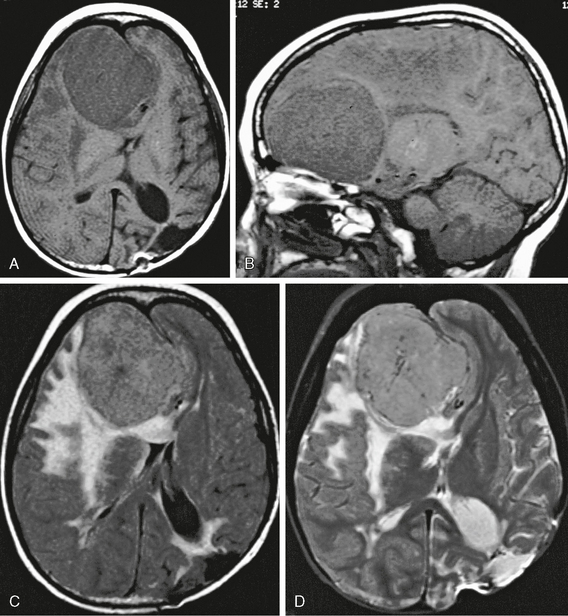
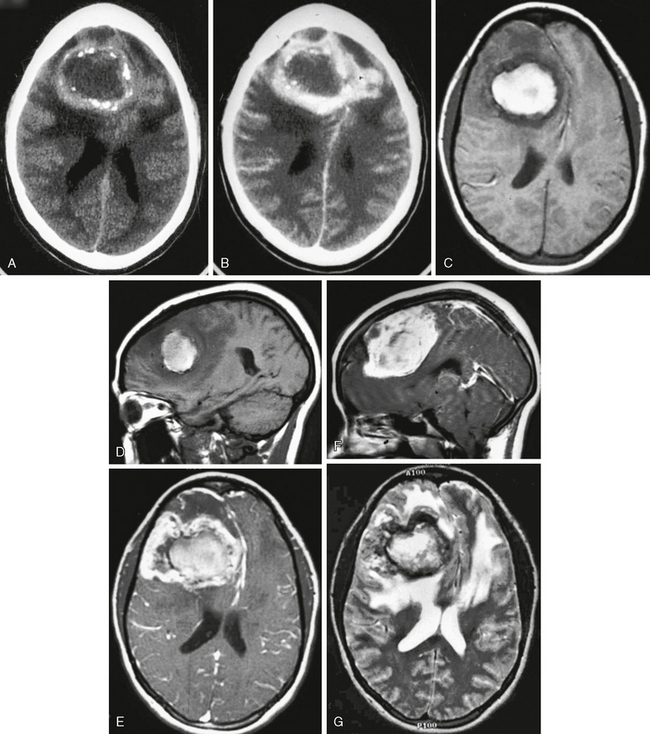
FIGURE 30-7 Olfactory groove meningioma. A 13-year-old girl with a history of previous left parietal peripheral neuroectodermal tumor and subsequent chemotherapy and radiation therapy presented with a headache that had been increasing in severity over the previous 2 weeks. Axial and sagittal T1W MR images (A, B) show a large extra-axial mass centered in the anteroinferior left frontal region with significant midline shift. Axial FLAIR (C) and T2W MR (D) images show prominent perilesional edema as well as postsurgical encephalomalacia in the left parietal lobe. Surgical excision followed by pathologic evaluation revealed olfactory groove meningioma with significant atypia classified as WHO grade II.
If any of several less common histologic variants are present, the MRI signal characteristics may vary, following water (Fig. 30-8), fat, or bone signal intensity. Central sand-like or psammomatous calcification is relatively common and will be hypointense on MRI (Fig. 30-9). Markedly prominent vascularity in an otherwise benign meningioma may be designated as an angiomatous meningioma (Fig. 30-10).
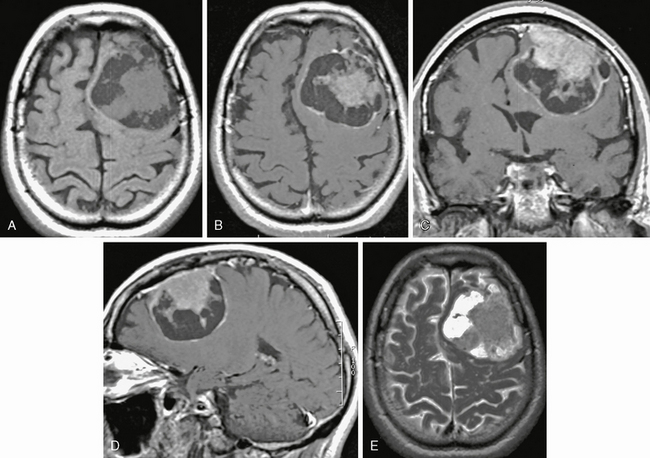
FIGURE 30-8 Cystic meningioma. Axial T1W noncontrast (A) and axial (B), coronal (C), and sagittal (D) postgadolinium and axial T2W (E) MR images show left frontal meningioma with prominent cystic regions typically located deep to the dural surface.
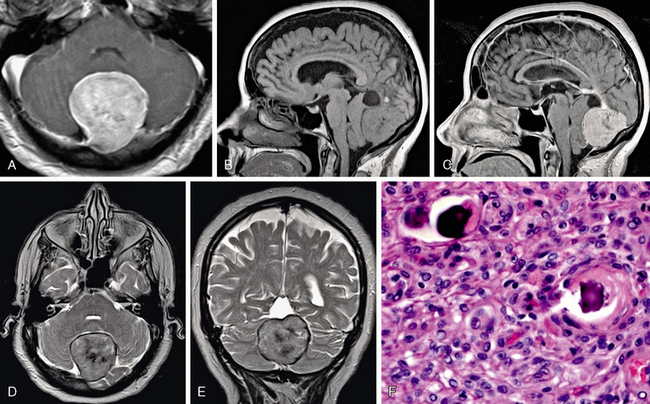
FIGURE 30-9 Psammomatous meningioma. Axial T1W postgadolinium MR image (A), sagittal pre- and postgadolinium MR images (B, C), and axial and coronal T2W MR images (D, E) show hazy low signal intensity, as well as a dural tail in this posterior fossa meningioma. The histologic slide (F) shows the sand-like calcification or psammoma body.
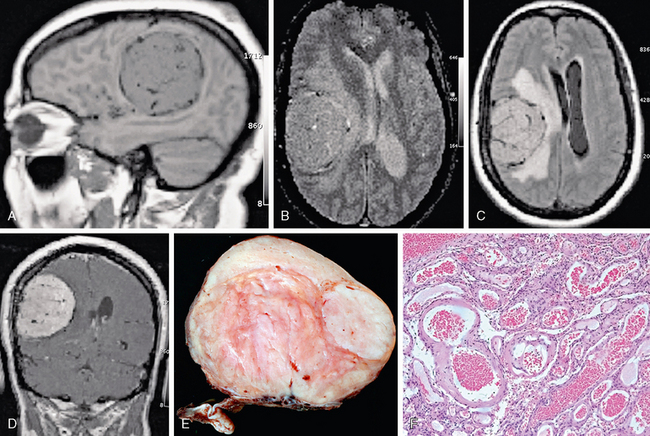
FIGURE 30-10 Angiomatous meningioma. A 53-year-old woman presented with left upper extremity symptoms. Sagittal T1 (A), axial T2 (B), FLAIR (C), and coronal postgadolinium T1W (D) MR images display a highly vascular right frontoparietal mass with dense homogeneous enhancement with prominent vascular flow voids. Gross (E) and microscopic (F) pathologic sections also show an unusually prominent vascular pattern.
Morphologic variations range from rounded to flattened, or en plaque, configurations. Perilesional edema may be present to varying degrees, and some studies suggest that this is more common in frontal than occipital tumors. Although present in all three grades, perilesional edema may be somewhat more common and more exuberant in higher-grade tumors and may be associated with increased likelihood of local recurrence after surgical resection (Fig. 30-11).
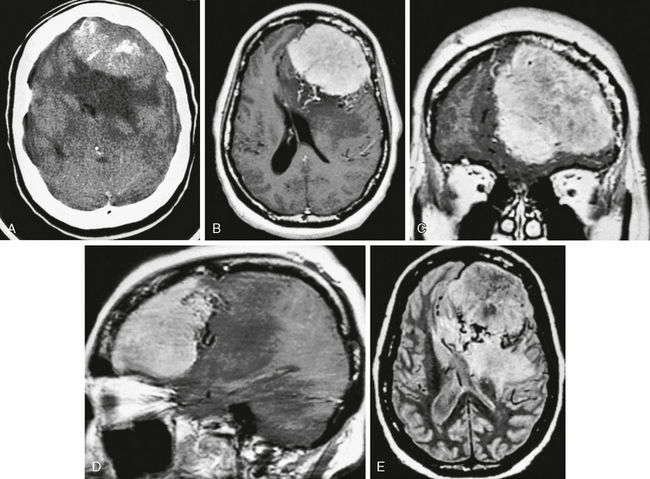
FIGURE 30-11 Malignant meningioma, WHO grade III. This tumor shows increased density on a nonenhanced CT image (A) and diffuse relatively homogeneous enhancement on postgadolinium axial (B), coronal (C), and sagittal (D) T1W and axial proton density (E) MR images. Note the irregular margins, prominent vascularity, and exuberant perilesional edema, all of which are nonspecific but atypical for benign meningioma.
Meningioma location is usually dural based but may also be intraventricular, usually in the atrium of the lateral ventricles. Intraventricular meningiomas are most commonly typical grade I benign lesions (Fig. 30-12) but may occasionally be atypical grade II (Fig. 30-13) or even malignant grade III lesions.
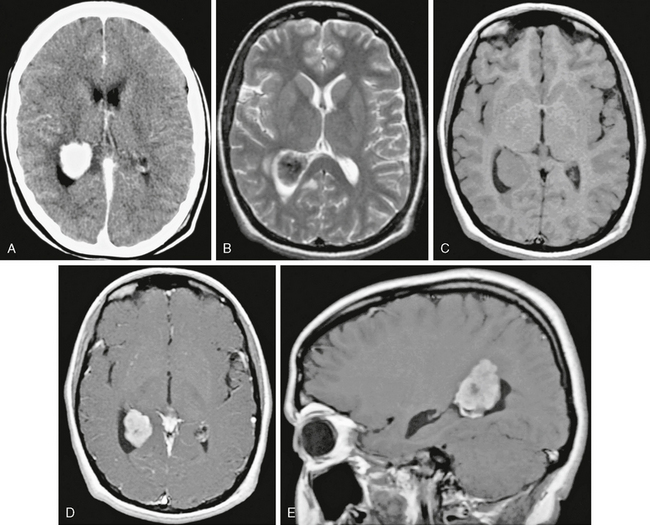
FIGURE 30-12 Intraventricular meningioma, WHO grade I, in a 16-year-old girl with recurrent syncope. Contrast-enhanced CT image (A) shows an ovoid, well-circumscribed, densely enhancing mass in the atrium of the right lateral ventricle. Axial T1W (B), T2W (C), and axial and sagittal postgadolinium (D, E) MR images show a densely enhancing mass with some low signal intensity on T2 weighting, suggesting calcification. Pathologic examination revealed typical meningothelial meningioma.
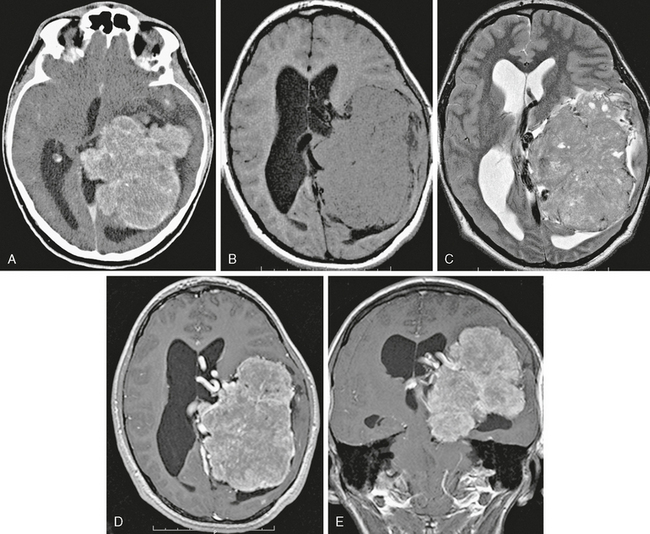
FIGURE 30-13 Intraventricular meningioma, WHO grade II. Axial contrast-enhanced CT image (A) and axial T1W (B), T2W (C), and postgadolinium axial (D) and coronal (E) T1W MR images show a large mass centered at the atrium of the left lateral ventricle with somewhat irregular margins and diffuse but slightly heterogeneous enhancement. Atypical imaging features correspond, in this case, but not always, with atypical histology.
Atypical (grade II) meningiomas, such as clear cell (Fig. 30-14) and chordoid (Fig. 30-15) subtypes, or malignant (grade III) meningiomas, including subtypes papillary (Fig. 30-16) and rhabdoid (Fig. 30-17), may be indistinguishable from “vanilla” or grade I tumors. Not uncommonly, however, they may display greater irregularity in margins and in overall morphology and may be seen to invade brain parenchyma. Florid perilesional edema is somewhat more common in higher-grade meningiomas as well. Atypical imaging features do sometimes but not invariably follow atypical histologic patterns.
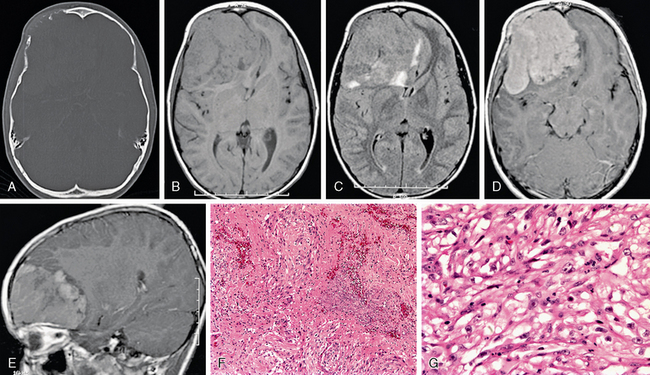
FIGURE 30-14 Meningioma, clear cell subtype, WHO grade II. An 11-year-old boy presented with a palpable abnormality of the right forehead. CT with bone windows (A) demonstrates bone invasion and destruction. Axial T1 (B) and FLAIR (C) MR images show a large, somewhat irregular mass that is slightly heterogeneous with perilesional edema. Axial (D) and sagittal (E) postgadolinium T1W MR images show moderate, diffuse, but somewhat heterogeneous enhancement. Photomicrographs taken after staining with hematoxylin and eosin at 100× (F) and 400× (G) show meningothelial cells with round to ovoid nuclei, multiple prominent nucleoli, and clear cytoplasm. Multiple mitotic figures are seen as well as foci of necrosis, thereby meeting criteria for WHO grade II.
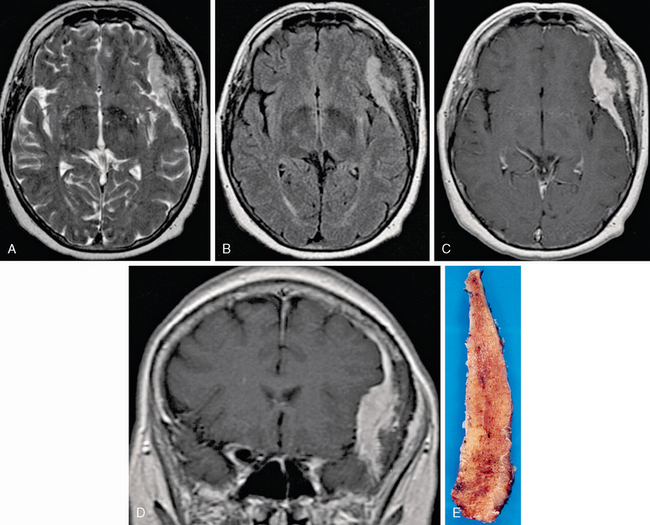
FIGURE 30-15 Intraosseous meningioma, chordoid subtype, WHO grade II, in a 65-year-old woman with confusion, amnesia, and a palpable left temporal lesion. Axial T2W (A), FLAIR (B), and axial (C) and coronal postgadolinium T1W (D) MR images reveal expansion of the temporal bone (E) with an en plaque–enhancing dural lesion and extension into scalp and temporalis muscle. Pathologic examination reveals “nests and cords of cells embedded in a mucoid matrix” with a moderate mitotic index, as well as extensive invasion of scalp and dura.