CHAPTER 40 Meningitis and Ventriculitis
BACTERIAL MENINGITIS
Epidemiology
In the United States, bacterial meningitis is predominantly a disease of adults.1–3 The most common (50%) cause of adult meningitis is Streptococcus pneumoniae. Other common causative organisms include Neisseria meningitidis and Haemophilus influenzae. Gram-negative bacillary meningitis shows an increased incidence in patients who have undergone neurosurgical procedures.3 Successful vaccination against H. influenzae type B has markedly reduced the incidence of this infection in infants and children, leaving group B streptococci as the most common cause of bacterial meningitis and sepsis in the neonate. Group B streptococci are also an infrequent cause of meningitis in adults, especially in patients with diabetes mellitus, liver disease, prior stroke, breast cancer, and human immunodeficiency virus (HIV) infection.2 Citrobacter is a distinct group of gram-negative bacilli that belong to the Enterobacteriaceae family. Citrobacter meningitis is frequent in neonates and young children but highly unusual in adults.
Pathology
Congestion and hyperemia of the leptomeninges with exudates in the subarachnoid space are typical pathologic findings in meningitis.4
Imaging
CT
When meningitis is suspected clinically, CT is usually obtained before lumbar puncture to exclude a mass lesion or other signs of elevated intracranial pressure. Noncontrast CT is often normal in early cases of uncomplicated meningitis.5 Later, the meninges may show contrast enhancement, suggesting inflammation. However, meningeal enhancement alone is not specific for meningitis (Fig. 40-1).
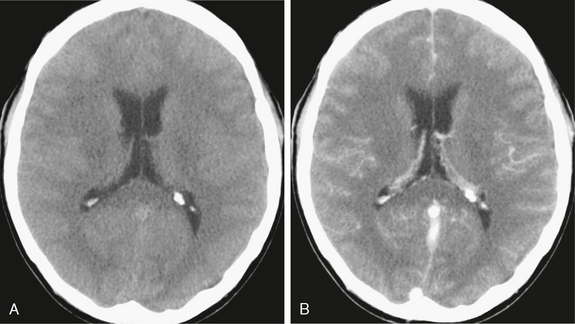
FIGURE 40-1 Bacterial meningitis. A 35-year-old man presented with headache and neurologic signs of meningeal disease. A, Axial noncontrast scan shows obliteration of the sulci over the hemispheres. No enlargement of the ventricles was observed. B, Subtle meningeal enhancement was noted on contrast-enhanced CT scan.
MRI
Fluid-attenuated inversion recovery (FLAIR) MR sequences appear to be the most sensitive technique for detecting meningeal diseases (Fig. 40-2). High signal in the subarachnoid space reflects high protein concentration in the CSF.5–7 Contrast-enhanced T1-weighted (T1W) images typically show leptomeningeal enhancement (Fig. 40-3).
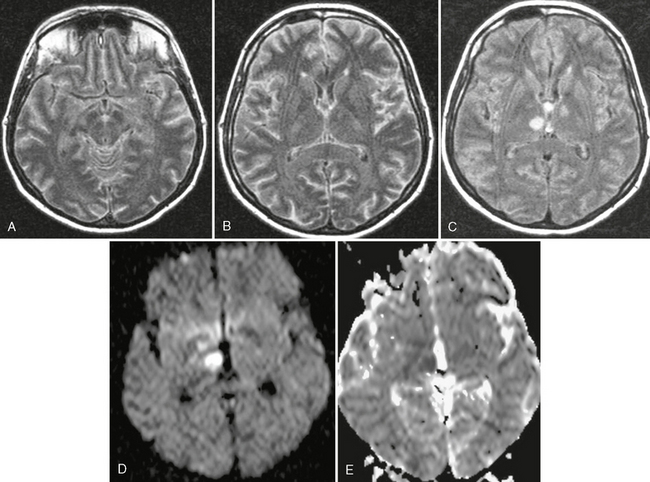
FIGURE 40-2 Streptococcus pneumoniae meningitis in a 40-year-old woman. A to C, On axial FLAIR MR image (TR/TE/TI 10,000/130/2,100), high signal intensity is shown in the subarachnoid spaces, indicating a meningeal process. In addition, rounded hyperintensities were seen in both thalami (C). The trace DWI (D) and the ADC map (E) showed high signal with low ADC values, consistent with arterial infarctions and cytotoxic edema.
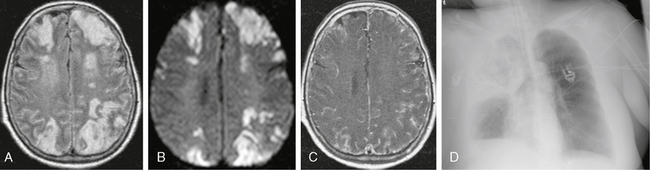
FIGURE 40-3 Pneumococcal meningoencephalitis in a 50-year-old patient with pneumonia, fever, and seizures. A, Axial FLAIR-TSE MR image (TR/TE/TI 7385/130/2100) shows bilateral, symmetric, high signal intensity in the cortex and underlying white matter. B, Trace DWI shows high signal with low ADC values (not shown), indicating restricted diffusion. C, T1W contrast-enhanced MR image (TR/TE/flip° 20/2.1/35°) with magnetization transfer shows marked meningeal enhancement. D, Typical pattern of a lobar pneumonia was detected on chest radiography.
Communicating hydrocephalus is a common complication, because the inflammatory debris may impede the flow and reabsorption of CSF.8 The ventricles become distended. Transependymal “migration” of CSF appears as high signal intensity areas surrounding the portions of the ventricles that abut white matter. Pyogenic ventriculitis manifests as periventricular high signal on FLAIR MRI, ependymal enhancement, ventricular debris, and fluid-fluid levels within the ventricles.9 Subdural effusions are typically sterile in meningitis; only 2% will form subdural empyemas. Venous thrombosis appears as high signal intensity within the venous sinuses on spin-echo sequences and absence of high signal within the sinuses on gradient-echo sequences. Such thromboses can lead to venous infarctions that do not conform to well-defined arterial territories and often manifest concurrent hemorrhage. Venous infarctions typically show high signal on T2-weighted (T2W) and FLAIR images as well as high signal on diffusion-weighted imaging (DWI) with low apparent diffusion coefficient (ADC). They are usually located cortically/subcortically close to the vertex and the thrombosed superior sagittal sinus. Occlusions or stenosis of the arteries may be detected on magnetic resonance angiography (MRA). Arterial infarctions have typical MR features and follow the expected arterial distributions (see Fig. 40-2).
EMPYEMA
Epidemiology
Purulent subdural effusions occur most frequently in the second decade of life with a male-to-female ratio of 3 : 1.10 The most common pathogens are Streptococcus milleri, other streptococci, enterococci, and gram-negative bacilli. Most subdural empyemas develop as complications of sinusitis, then otitis media. In one study, 6% of patients with otitis media had a subdural or epidural empyema.11 Infratentorial empyema is an uncommon form of intracranial suppuration and is usually secondary to a neglected otogenic infection.12 Only 41 cases of infratentorial empyema were described in the literature from 1966 through 2006.12
Clinical Presentation
Subdural empyema presents clinically as seizures, focal deficits, and progressive neurologic deterioration. Fever, vomiting, and meningismus may suggest meningitis. The clinical course is usually fulminant, with rapid development of coma. If the empyema is located in the frontal lobe, the only clinical signs may be subtle changes in personality or mood without focal neurologic symptoms. The clinical presentation of infratentorial empyema includes meningismus, altered level of consciousness, signs of raised intracranial pressure, draining ear, and pyrexia.12 Compared with subdural empyema, epidural empyema presents in a more subtle fashion, usually with several days of fever along with mental status changes and neck pain.13
Pathophysiology
Epidural empyemas tend to remain localized within the extradural space. Subdural empyemas commonly spread diffusely over the convexities and throughout the subdural space, because no anatomic constraints limit their spread.10 Subdural empyema usually occurs in association with otorhinologic infection as a result of direct spread of infection or retrograde thrombophlebitis via bridging emissary veins. Because the bridging veins are valveless, thrombophlebitis can easily pass retrograde into the cavernous sinus and other dural venous sinuses. In the acute phase, a thin unencapsulated layer of pus covers the cerebral hemisphere. Retrograde thrombophlebitis produces early involvement of the cortex.
Imaging
Ultrasonography
Ultrasonography can be used only in small infants when the fontanelles are still open.
CT
Subdural Empyema
Subdural empyema appears as a low-density subdural lesion on noncontrast CT and shows thick-walled marginal dural enhancement on contrast-enhanced CT.14 CT shows displacement of the cortical surface, deflection of the gray matter/white matter interface (buckled white matter sign), deflection of the surface veins, and, perhaps, air bubbles within the collection. If sinusitis is present, the sinuses may appear opacified, with air-fluid levels and, perhaps, bony erosion. Venous stasis and thrombosis may produce hyperemia, which manifests as cortical swelling and minimal hyperdensity on noncontrast CT and as diffuse gyral enhancement on contrast-enhanced CT.
MRI
Subdural empyema is usually crescentic, whereas epidural empyema is always lentiform. The pus within the subdural/epidural empyema is hypointense relative to the brain on T1W and hyperintense on T2W images. FLAIR MRI helps to distinguish infected effusions (high signal) from sterile hygromas (low signal) (Fig. 40-4A). The dura mater shows characteristically low signal on both T1W and T2W images, so identification of the low signal dura will help to distinguish a subdural empyema from an epidural empyema on both T1W and T2W imaging. On DWI, purulent subdural collections will show high signal due to restricted diffusion (Fig. 40-5D).15 Epidural empyemas may show high, low, or inhomogeneous signal on DWI. The difference in pressure and other factors between the subdural and epidural spaces, as well as the content of empyemas, may be the causes of differences in the activity of pleomorphic leukocytes.
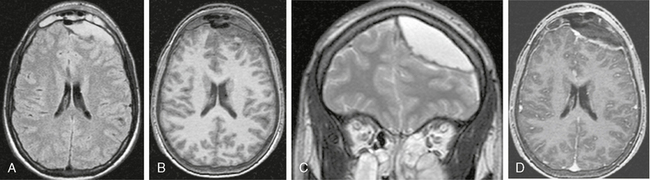
FIGURE 40-4 Epidural empyema in a patient with left frontal sinusitis. A, Axial FLAIR-TSE MR image (TR/TE/TI 7385/130/2100) shows a hyperintense, lenticular lesion in the epidural space overlying the left frontal lobe. Medially displaced dura is recognized as a low signal intensity line between the lesion and the brain parenchyma. The left frontal sinus is opacified. The empyema is hypointense on an axial, precontrast T1W image (B) and hyperintense on a coronal T2W image (C). Gadolinium-enhanced T1W image (TR/TE/flip° 20/2.1/35°) shows marked enhancement of the dura with clear delineation of the epidural empyema (D).
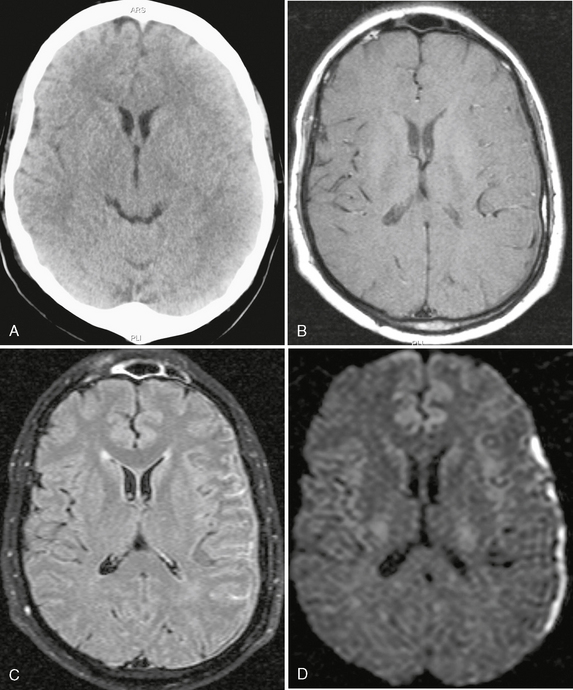
FIGURE 40-5 Bacterial meningitis with subdural empyema. A, No abnormality was noted on the noncontrast CT of the brain. B, On a precontrast T1W image (TR/TE/flip° 20/2.1/35°), the dura is shown on the left side as a hypointense line. C, On axial FLAIR-TSE MR image (TR/TE/TI 7385/130/2100), high signal is demonstrated in the left subarachnoid spaces and in the subdural space overlying the left hemisphere. D, On trace DWI, the subdural collection has high signal, indicating restricted diffusion within purulent fluid: a subdural empyema.
PYOGENIC PARENCHYMAL INFECTIONS
Cerebritis
Cerebritis is a purulent nonencapsulated parenchymal infection of the brain.
Epidemiology
The frequency of focal infections of the brain parenchyma varies among series, with a highest reported frequency of 19%.16



