CHAPTER 11 Mesenteric arteries
Arteriography (online videos 11-1 to 11-3)
A wide variety of catheters are available for mesenteric arteriography; the Sos and cobra shapes are popular (see Figs. 3-14 and 3-15). The celiac and superior mesenteric arteries are engaged by searching the anterior aortic wall at about the T12 and L1 levels, respectively. The inferior mesenteric artery (IMA) typically originates from the left anterolateral surface of the aorta at about the L3-L4 level. If the desired vessel is not easily found, aortography will often show an obstructed origin or atypical location. Inadvertent dissection of a mesenteric arterial trunk or branch can have serious consequences. Therefore great care should be taken with catheter and guidewire manipulations. Large contrast injections are only made after secure intravascular location is documented and good backflow of blood is observed.
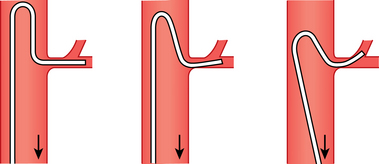
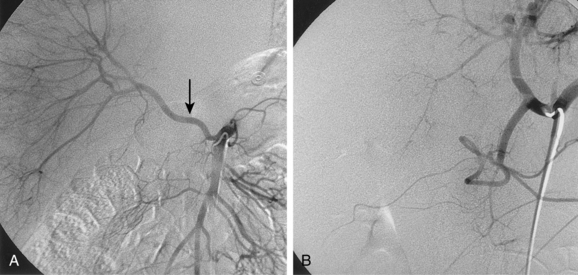
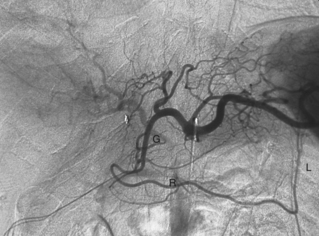
Most practitioners use coaxial microcatheters and microwires for selective catheterization of mesenteric artery branches. Occasionally, a larger catheter or sheath must be negotiated into a second- or third-order vessel to allow an intervention. In this situation, the catheter may be advanced using steerable, hydrophilic guidewires or an exchange done with a more suitably shaped catheter and wire. There are several methods for engaging the left gastric artery (LGA); one traditional technique is outlined in Figure 11-1.
Anatomy (online cases 6 and 19)
Development
In the fetus, a series of vitelline arteries arising from the fused dorsal aortae supply the abdominal viscera.1 These branches are initially connected by a ventral anastomotic channel (Fig. 11-2). The celiac, superior mesenteric, and inferior mesenteric arteries originate from three of these vitelline segments; the remaining segments regress before birth. Common variants in mesenteric artery anatomy can be explained by abnormal persistence of vitelline remnants or portions of the ventral anastomosis.
Normal anatomy
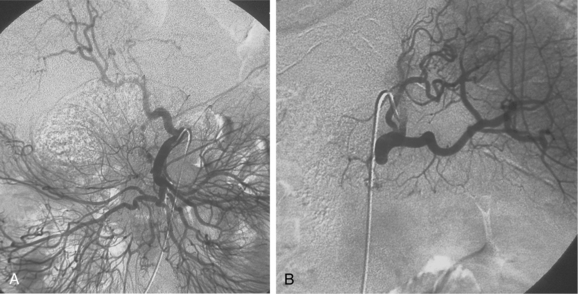
The celiac artery exits the anterior surface of the aorta at about the T12 level2–4 (Fig. 11-3). The main trunk can take an upward, downward, or forward course. Just beyond its origin, the celiac artery gives off the LGA and then divides into the common hepatic and splenic arteries. Sometimes, the inferior phrenic or dorsal pancreatic artery arises directly from the celiac trunk.
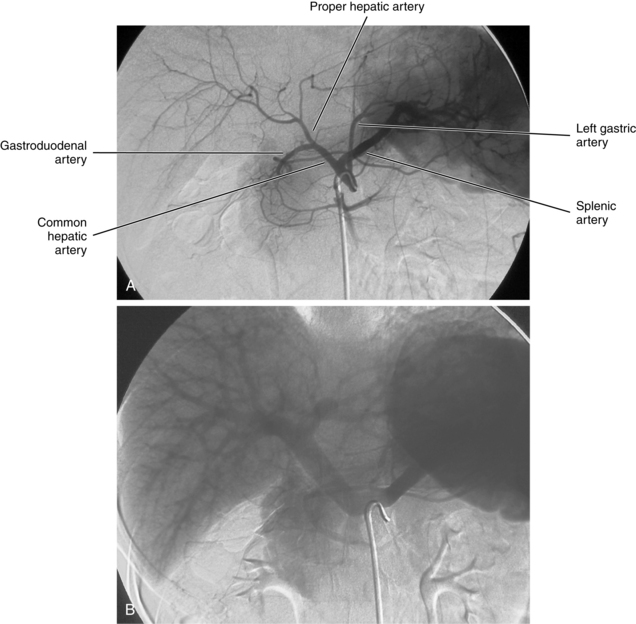
Figure 11-3 Celiac arteriogram. A, Arterial phase. B, Venous phase with filling of the splenic and portal veins.
The common hepatic artery runs toward the liver. After giving off the gastroduodenal artery (GDA), it becomes the proper hepatic artery, which itself divides into right and left (and occasionally middle) hepatic arteries. Hepatic arterial anatomy is detailed in Chapter 12. The right gastric artery usually takes off from the proper or left hepatic artery but sometimes is not visualized on nonselective angiograms. It supplies the pylorus and the lesser curvature of the stomach and communicates with distal branches of the LGA. The cystic artery usually originates from the right hepatic artery, although it may arise more proximally.
The GDA runs between the neck of the pancreas and the duodenum. Its first major branch is the posterior superior pancreaticoduodenal artery. This vessel gives off branches to the pancreas on the left and to the duodenum on the right. The GDA then divides into its terminal branches, the anterior superior pancreaticoduodenal artery and the right gastroepiploic artery.The superior pancreaticoduodenal arteries lead into an arterial network that supplies the head of the pancreas. These vessels have rich anastomoses with the corresponding branches of the inferior pancreaticoduodenal artery, which originates from the superior mesenteric artery (SMA). The right gastroepiploic artery runs along the greater curvature of the stomach and communicates with the left gastroepiploic branch of the splenic artery.
The splenic artery supplies the spleen, pancreas, and stomach (see Chapter 12). It runs dorsal to the upper border of the pancreas along with the splenic vein. Its branches usually include the dorsal pancreatic, pancreatica magna, short gastric, and polar arteries. The splenic artery divides into superior and inferior branches near the splenic hilum. The inferior polar artery gives rise to the left gastroepiploic artery, which courses along the greater curvature of the stomach.
The LGA exits the upper surface of the celiac artery and runs toward the cardia of the stomach (Fig. 11-4). At this point, it divides into several branches that supply the distal esophagus and fundus of the stomach. These vessels communicate with short gastric branches of the splenic artery, the right gastric artery, and the left inferior phrenic artery.
The SMA supplies the bowel from the distal duodenum to the mid-transverse colon (Fig. 11-5). It courses behind the body of the pancreas and then enters the root of the mesentery. The superior mesenteric vein runs along the right side of the artery. The inferior pancreaticoduodenal artery is the first branch of the SMA, although it may arise in a common trunk with the first jejunal artery. It passes to the right and superiorly into the head of the pancreas, where it communicates with the superior pancreaticoduodenal branches of the GDA. A series of jejunal and ileal branches occupy most of the main trunk of the SMA. Usually, these vessels fan out from the left upper quadrant to the right lower quadrant. A system of elaborate arcades between these branches feed the vasa recta that directly supply the wall of the bowel. The ileocolic artery is the terminal continuation of the SMA. It gives off branches to the ileum, appendix, cecum, and right colon. The right and middle colic arteries arise separately or as a common trunk on the right side of the SMA. These vessels supply the ascending and transverse colon, respectively. Small bowel branches of the SMA can be differentiated from colonic branches by their more extensive arcade system and longer vasa recta (see Fig. 11-5).
The IMA supplies the colon from its mid-transverse segment to the rectum (Fig. 11-6). The vessel runs inferiorly for several centimeters and then gives off the left colic artery. An ascending branch of this artery joins the middle colic branch of the SMA. Descending colic (sigmoid) branches originate from the left colic artery and distal IMA to supply the lower descending and sigmoid colon. The terminal branch of the IMA is the superior rectal (hemorrhoidal) artery, which divides into right and left branches that supply blood to the rectum.
Variant anatomy
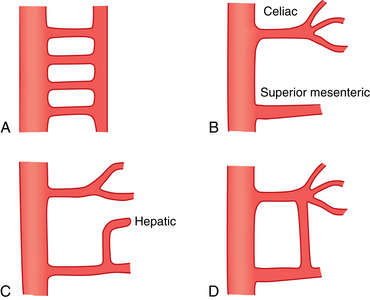
Anomalies in the origins of the central mesenteric arteries are rare, but anomalies in the branching patterns of the mesenteric arteries are common. It is vital to be alert to potential variants when performing angiographic procedures. Table 11-1 outlines the important variants formulated by the classic anatomic studies of Michel and coworkers5,6 (Figs. 11-7 through 11-9; see also Fig. 7-4). The reported frequencies vary somewhat in postmortem and angiographic studies.
Table 11-1 Important Anomalies of the Mesenteric Arteries
| Artery | Anomalous Origin | Frequency (%) |
|---|---|---|
| Replaced common hepatic | Superior mesenteric | 2.5 |
| Replaced right hepatic | Superior mesenteric | 10 |
| Accessory right hepatic | Superior mesenteric | 6 |
| Replaced left hepatic | Left gastric | 10 to 12 |
| Accessory left hepatic | Left gastric | 8 to 13 |
| Inferior phrenic | Celiac | 35 |
| Right gastric | CHA, RHA, GDA | 15 |
| Dorsal pancreatic | Celiac | 22 |
| Gastroduodenal | Right or left hepatic | 18 |
| Left gastric, splenic, hepatic | Aorta | <1 |
| Celiomesenteric trunk | Aorta | <1 |
| Middle colic | Dorsal pancreatic, splenic, hepatic | <1 |
CHA, common hepatic artery; GDA, gastroduodenal artery; RHA, right hepatic artery.
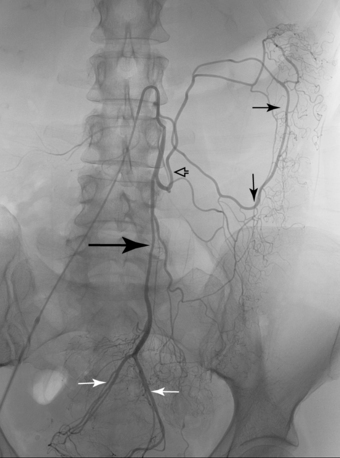
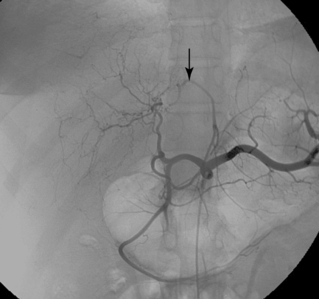
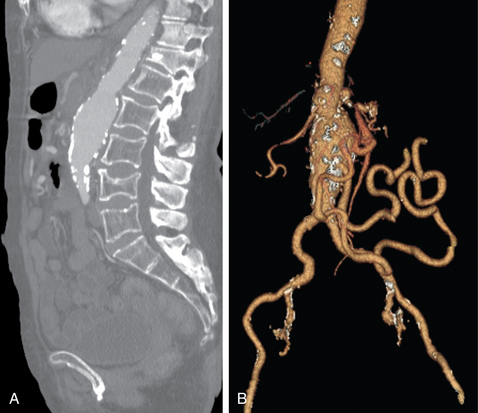
Other rare variants include common origin of the celiac artery and SMA (celiomesenteric trunk,Fig. 11-10), separate origin of one or more major celiac branches from the aorta, and splenic artery arising from the SMA (Figs. 11-11 and 11-12).
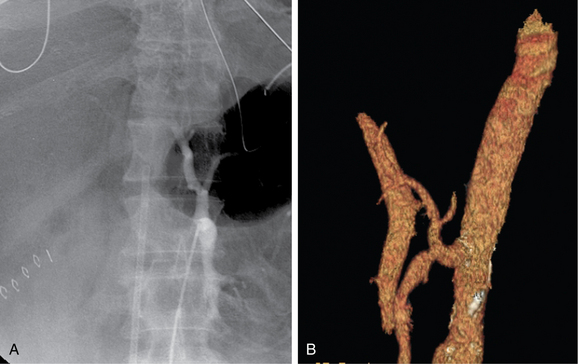
Figure 11-10 Common celiomesenteric trunk on mesenteric arteriography (A) and sagittal shaded surface display CT angiography (B).
Collateral circulation
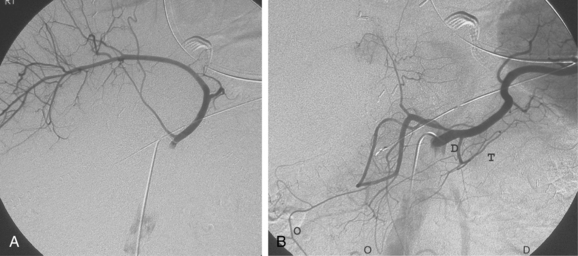
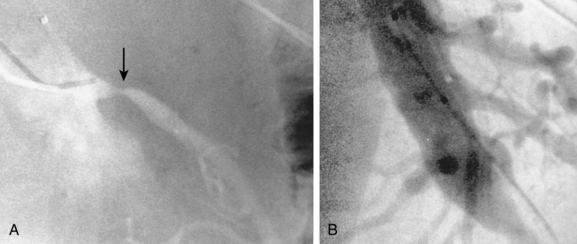
The intestinal tract has an extensive collateral network that prevents or limits bowel ischemia when central arteries become obstructed. The more important pathways6 are outlined in Table 11-2 and shown in Figures 11-13 through 11-16. The different collateral routes between the SMA and IMA are often confused. The marginal artery (of Drummond) is a longitudinal vessel formed by trunks or distal branches of the right, middle, and left colic arteries. When fully formed, it runs along the entire mesenteric border of the colon. The arc of Riolan is a central vessel embedded in the root of the mesentery that directly connects the middle colic artery with the left colic artery.
Table 11-2 Major Mesenteric Arterial Collateral Pathways
| Pathway | Communication |
|---|---|
| Celiac and Superior Mesenteric Arteries (seeFig. 11-13) | |
| Pancreatic arcade | Gastroduodenal and inferior pancreaticoduodenal arteries |
| Arc of Buehler | Direct embryologic pathway |
| Intraceliac Branches (seeFig. 11-14) | |
| Gastric arcade | Left and right gastric arteries |
| Gastroepiploic arcade | Left and right gastroepiploic arteries |
| Arc of Barkow | Left and right epiploic arteries (within omentum) |
| Superior and Inferior Mesenteric Arteries (seeFig. 11-15) | |
| Marginal artery | Branches of middle and left colic arteries (of Drummond) |
| Arc of Riolan | Central vessel from middle to left colic artery |
| Inferior Mesenteric and Iliac Arteries | |
| Rectal (hemorrhoidal) | Superior and middle/inferior rectal arteries arcade |
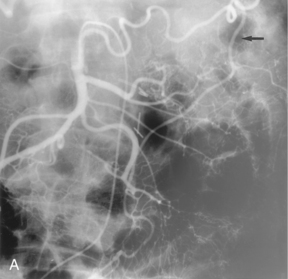
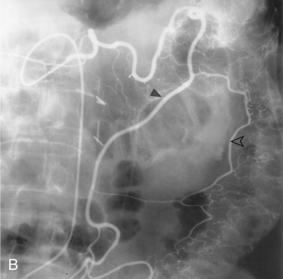
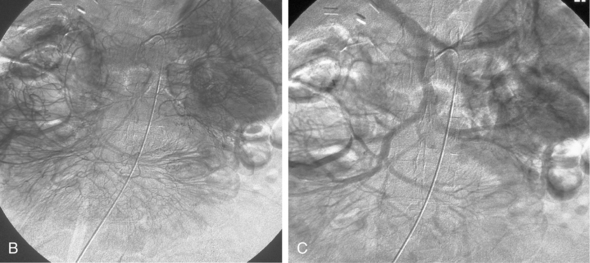
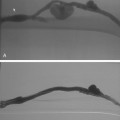
Figure 11-15 Marginal artery of Drummond and arc of Riolan with inferior mesenteric artery (IMA) occlusion. A, Superior mesenteric arteriogram shows dilation of the middle colic artery with flow into the IMA distribution (arrow).B, Selective middle colic artery injection shows filling of the arc of Riolan (arrowhead) and the marginal artery (open arrowhead).
Major disorders
Acute mesenteric ischemia
Etiology
It is noteworthy that sudden obstruction of the celiac artery or IMA rarely causes symptomatic mesenteric ischemia, as long as adequate collaterals exist. Several vascular diseases that reduce blood flow to or from the intestinal arteries or veins can lead to acute mesenteric ischemia7–11 (Box 11-1). SMA embolism is one of the most common causes of acute mesenteric vascular obstruction. Most emboli come from the heart. The embolus typically lodges in the SMA just below the origin of the middle colic artery, although single or multiple emboli may pass into the distal SMA trunk or its branches. The arteries beyond the obstruction undergo reflex vasoconstriction, which further worsens bowel ischemia. SMA thrombosis is another frequent cause for acute intestinal ischemia. Thrombosis is usually superimposed on severe atherosclerotic disease near the origin of the SMA.
Nonocclusive mesenteric ischemia is responsible for some cases of bowel ischemia.11–12 The disorder is caused by hypotension or hypovolemia in the setting of acute cardiac disease, sepsis, liver or kidney disease, and after recent surgery. Certain drugs (including digitalis, dopamine, and vasopressin) have also been implicated. Regardless of the cause, the result is persistent vasoconstriction of the SMA and its branches leading to intestinal hypoxia. This entity is particularly lethal; the 30-day mortality rate despite surgery in one large contemporary series was 80%.13
Superior mesenteric vein thrombosis may be spontaneous or secondary to portal hypertension, thrombophilic states, trauma, abdominal surgery, inflammatory bowel disease, sepsis, neoplasm, or pancreatitis.14 If the venous collateral circulation is inadequate, the bowel wall becomes edematous, and arterial inflow is diminished. Finally, extension of an aortic dissection into main mesenteric trunks is an important reason for inadequate perfusion of bowel.
Clinical features
Most individuals with acute mesenteric ischemia are older adults. Many have associated conditions such as congestive heart failure, coronary artery disease, valvular heart disease, dysrhythmias, or hypotension. Patients usually present with acute onset of moderate to severe abdominal pain, sometimes accompanied by nausea and vomiting or gastrointestinal bleeding. Classically, the symptoms are out of proportion to the relatively benign findings on physical examination. Often, a leukocytosis and metabolic acidosis are present. If bowel infarction occurs, signs of an acute abdominal condition will develop. Prompt diagnosis is important to avoid irreversible bowel damage, perforation, and sepsis, which usually occurs within 12 to 24 hours of occlusion unless the collateral circulation is already well developed. If the condition is left untreated, the mortality rate can be as high as 60% to 100%.15,16
Imaging
Cross-sectional techniques
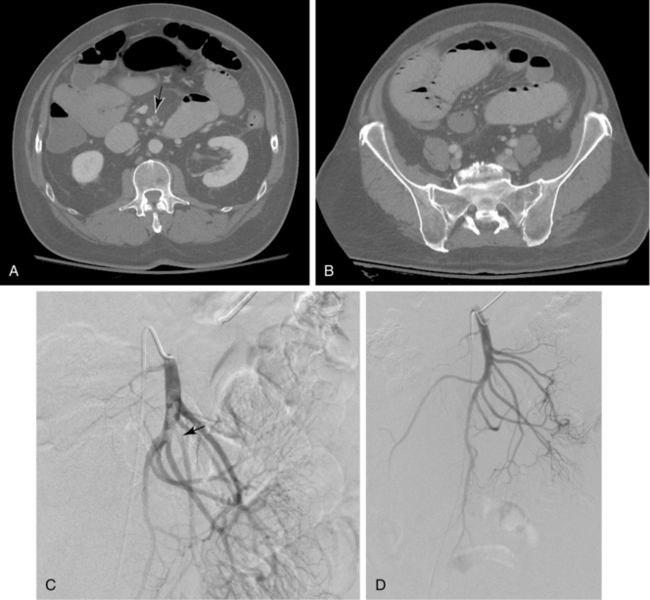
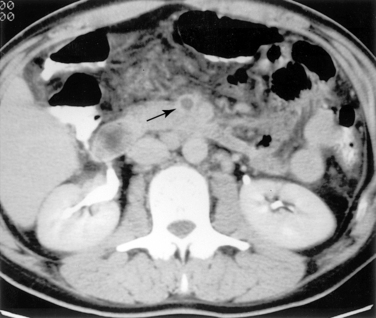
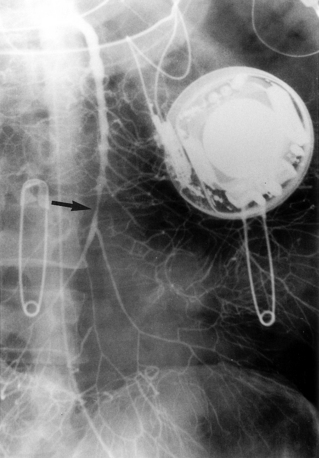
Duplex ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) can play an important role in diagnosis and are particularly useful for excluding other sources of abdominal pain.17–21 Sonography is used primarily to assess the patency of the central mesenteric vessels. Findings on CT or MRI include bowel distention and wall thickening, edema of the mesentery, intramural gas, and, sometimes, diminished bowel wall enhancement. Filling defects in the major mesenteric arteries and veins should be carefully sought (Figs. 11-17 and 11-18).
Catheter angiography
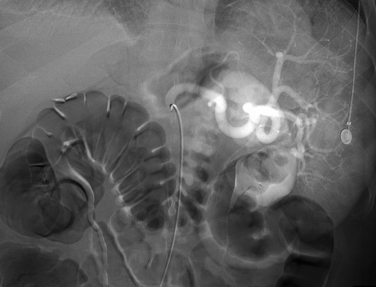
Arteriography is indicated when noninvasive imaging is equivocal, nonocclusive mesenteric ischemia is suspected, or endovascular treatment is contemplated. Arteriographic findings depend on the cause of occlusive disease. A fresh embolus produces a discrete filling defect (see Fig. 11-17). In some cases, residual flow is seen around the clot. Reconstitution of the distal SMA is usually poor or nonexistent. Occasionally, small clots are seen in distal SMA branches or other abdominal and pelvic arteries. Thrombotic occlusion causes complete obstruction in the most proximal portion of the SMA trunk (see Fig. 11-16). Because thrombosis usually occurs at a site of previous narrowing, the collateral circulation is often well developed. Despite certain distinguishing features, it may be impossible to differentiate thrombotic and embolic occlusions by angiography.
Nonocclusive mesenteric ischemia is characterized by slow flow in the SMA, diffuse narrowing of SMA branches and arcades, segments of alternating spasm and dilation of these branches, and poor filling of the vasa recta11,22 (Fig. 11-19). Contrast persists in intestinal branches for greater than 2 seconds after the injection has ended. Profound systemic hypotension (“shock bowel”) and certain vasoconstricting drugs can produce similar findings (see later discussion).
Treatment
Surgical therapy
Acute SMA occlusion is treated by embolectomy, thromboendarterectomy, patch angioplasty, or bypass grafting along with resection of infarcted bowel.7,8 Many patients still require a “second look” operation to identify additional ischemic bowel that must be removed. Despite early and aggressive treatment, 30-day mortality is still close to 30%.23,24
Endovascular therapy
Infusion of the vasodilator papaverine directly into the SMA (60-mg bolus followed by an infusion at 30 to 60 mg/hr) is sometimes effective as primary treatment for nonocclusive mesenteric ischemia or as a preoperative maneuver for other forms of acute mesenteric ischemia.22 Thrombolysis has also been used effectively for acute SMA embolism and acute superior mesenteric venous thrombosis25,26 (see Fig. 11-17). It may be particularly appropriate in patients without evidence of frank bowel necrosis. Balloon angioplasty with stent placement is also an attractive alternative to surgery in very high-risk patients with critical stenoses or extensions of dissection.27,28 Immediate clinical benefit is the rule, but long-term durability has not been established.
Chronic mesenteric ischemia (online case 67)
Etiology and clinical features
Symptomatic chronic obstruction of the mesenteric vessels is less common than acute intestinal ischemia. The primary cause of chronic obstruction is atherosclerotic disease8,10,14,15 (Box 11-2). Stenoses are often caused by plaques on the abdominal aortic wall that engulf the ostia of the mesenteric arteries. Subacute or chronic superior mesenteric vein thrombosis can mimic chronic arterial obstruction.
Imaging
Cross-sectional techniques
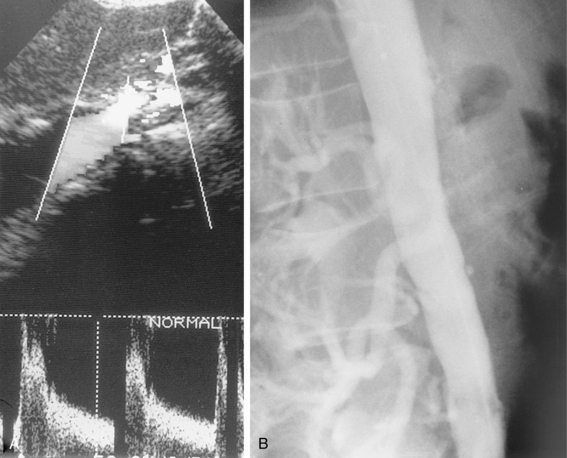
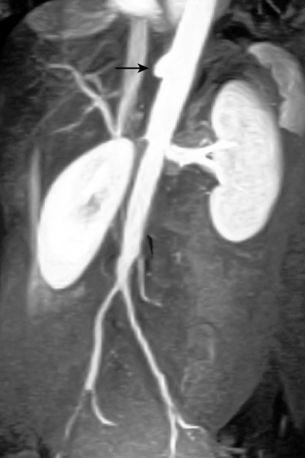
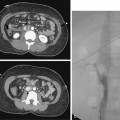
Because the symptoms of chronic mesenteric ischemia are often vague and intermittent, the diagnosis is often delayed. Duplex sonography is the primary imaging tool for screening in suspected cases. Criteria for diagnosis include major vessel occlusion, high-grade stenosis, or peak systolic velocities in the celiac and superior mesenteric arteries of greater than 200 cm/sec and greater than 275 cm/sec in the fasting state, respectively29–31 (Fig. 11-20). CT or MR angiography more thoroughly depicts mesenteric vascular stenoses and occlusions17,18 (Fig. 11-21). It is commonly accepted that at least two of the three mesenteric arteries must have significant stenoses or occlusions to make the diagnosis of chronic mesenteric ischemia. On the other hand, the mere presence of severe multivessel mesenteric artery disease can be an incidental finding in a patient with other causes for abdominal pain.
Catheter angiography
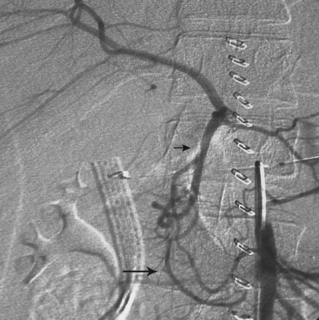
Arteriography is usually a prelude to percutaneous or open repair. Several entities should be considered in the differential diagnosis of mesenteric artery narrowing (see Box 11-2). Because of the chronic nature of the disease, the collateral circulation is usually extensive. Obstruction of the IMA is suggested by enlargement of the marginal artery (or arc of Riolan) with retrograde flow into the IMA trunk (see Fig. 11-15).
Treatment
Surgical therapy
The most common procedures for mesenteric revascularization are transaortic thromboendarterectomy, aortovisceral bypass, and arterial reimplantation.23,32
Endovascular therapy
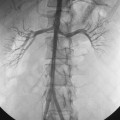
Balloon angioplasty with stenting of the celiac artery and SMA is an acceptable alternative to surgery in some cases, particularly when the patient is at high risk for operative complications8,32–36 (Fig. 11-22). Technical success is reported in 30% to 90% of cases. However, major complications occur frequently (16% to 33%) in some series.34 Surgery is more effective than endovascular repair; it affords more durable results and requires fewer reinterventions to maintain patency. Angioplasty and stent insertion is not useful in patients with celiac artery compression syndrome.
Acute gastrointestinal bleeding (online cases 32 and 103)
The diagnosis and treatment of acute gastrointestinal bleeding have undergone a revolution over the past several decades.37,38 The widespread use of histamine (H2) blockers and “proton pump” inhibitors has markedly reduced the incidence of peptic ulcer disease and associated bleeding. Fiberoptic endoscopy has largely supplanted angiography in the management of upper gastrointestinal hemorrhage.39 Upper endoscopy, colonoscopy, and push or double balloon enteroscopy have taken the dominant role in the evaluation and treatment of lower intestinal tract bleeding.40,41 Nonetheless, angiography is still required in a small subset of patients with this difficult clinical problem.