Miscellaneous Pulmonary Conditions: Inflammatory, Autoimmune, and Diseases of Unknown or Multifactorial Cause
John H. Juhl
Janet E. Kuhlman
J. H. Juhl and J. E. Kuhlman: Department of Radiology, University of Wisconsin Medical School, Madison, Wisconsin 53792-3252.
ATELECTASIS
General Considerations
Atelectasis is a state of incomplete expansion of a lung or any portion of it—ma loss of lung volume (collapse). There is a decrease or absence of air in the alveoli. The volume of the involved lung is therefore diminished. Atelectasis may be caused by bronchial obstruction or by extrapulmonary pressure by fluid, air, tumor, and the like. It is always a secondary lesion and is therefore a sign of disease rather than a disease in itself. Its causes can be grouped into six general categories.
Bronchial obstruction (resorption atelectasis) may be intrinsic, caused by tumor, foreign body, inflammatory disease, heavy secretions, and so forth. Extrinsic pressure on bronchi caused by tumor and enlarged nodes or bronchial constriction secondary to inflammatory disease may also cause it. In patients with airway obstruction, there is not always a decrease in volume caused by resorption of alveolar gas. Retained secretions may fill the bronchi and alveoli distal to the obstruction in some patients. In others, infection accompanied by exudate and transudate may fill the lung distal to the obstruction. The lung may therefore maintain its volume and increase in opacity. Also, collateral ventilation or air drift may occur through the pores of Kohn (in alveolar walls) or the canals of Lambert, which extend from preterminal bronchioles to alveoli. There may also be direct airway anastomoses allowing air drift. Therefore, air may be trapped distal to an airway obstruction and no collapse may occur. In collateral air drift, when air enters the obstructed segment more easily than it leaves, there may actually be an increase in lung volume (obstructive overinflation). When bronchial obstruction is acute, there is usually some replacement of the rapidly resorbed gas by a transudate (edema fluid), which may contain varying amounts of blood.
Atelectasis (passive) caused by space-occupying process that can compress the lung occurs in a variety of conditions, including pneumothorax, pleural fluid, diaphragmatic elevation (regardless of cause), herniation of abdominal viscera into the thorax, and large intrathoracic tumors.
Paralysis or paresis resulting in inability to expand a lung completely, as in poliomyelitis and other neurologic disorders, can also cause atelectasis. In addition to the weakness of the respiratory muscles per se, there is an inability to raise bronchial secretions, and this may add a factor of obstruction in these patients; however, the airways are generally patent.
Restriction of motion as a result of pleural disease or injury is another cause of atelectasis. An example is chronic constrictive pleuritis, which causes a decrease in volume of one hemithorax or a part thereof, so that normal expansion cannot occur. Acute conditions such as pleural infections and thoracic or upper-abdominal trauma may also restrict motion and therefore produce some degree of atelectasis. Patients may be unable to remove secretions, so an additional obstructive element may be present. Airways are usually patent, however.
Adhesive atelectasis refers to the nonobstructive airlessness found in patients with inactivation, decrease, or loss of surfactant. Hyaline membranes may then be formed within the alveoli, as in newborn respiratory distress syndrome, acute radiation pneumonitis, and uremia.
Cicatrization atelectasis refers to volume loss found in patients with local or general pulmonary fibrosis. Some prefer not to consider this a form of atelectasis, even though there is associated volume loss.
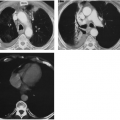
Chest radiographs are useful and reasonably accurate in patients with tumors causing bronchial obstruction and atelectasis, but when the cause of segmental or lobar atelectasis is not established with reasonable certainty on chest radiographs, computed tomography (CT) should be performed.109 Magnetic resonance imaging (MRI) may have a place in determining the cause of atelectasis based on differences in signal intensity.37
Radiographic Considerations
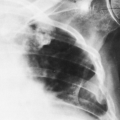
The fundamental alteration in the thorax produced by atelectasis is a decrease in volume of the segment, lobe, or lung involved. The interlobar fissure or fissures are displaced toward the airless lobe or segment. Crowding and displacement of vessels may be observed if they are surrounded by sufficient air-containing lung. Air bronchograms may be present, and bronchi may be displaced. There are other roentgen signs, such as elevation of the diaphragm, shift of mediastinum to the side of involvement, hilar displacement, and narrowing of rib interspaces. Any of these signs may predominate in a given instance, depending on mediastinal and diaphragmatic fixation. In some patients the remaining lung on the involved side undergoes so much compensatory overinflation that there is no actual change in volume of the hemithorax and few of the signs mentioned are then present. The relative airlessness of the affected portion of lung may result in very little increased density unless it is enhanced by edema, blood, and/or retained secretions. Therefore the amount of collapse does not necessarily correlate with the opacity of the involved lung. The classic ground-glass appearance is most likely caused by the opacity produced by collapsed lung which contains some fluid, plus air in a lobe or segment anterior or posterior to it. This appearance is noted most commonly in atelectasis of the left upper lobe. The opacity is uniform but has a grainy character that has been likened to the appearance of ground glass. It is usually relatively opaque medially and fades to a lesser opacity laterally. Wide variations in opacity are possible, however, depending on the relative amounts of aerated, collapsed, and fluid-filled lung. Disease in the involved lobe may also add to its opacity. When atelectasis involves the entire lung, the opacity may be complete and homogeneous. The bronchi as well as the lung may become airless in atelectasis caused by obstruction; then there is absence of an air bronchogram. If an air bronchogram is present, complete bronchial obstruction is unlikely. Absence of an air bronchogram does not necessarily mean obstruction, but does suggest it. Associated with mediastinal shift there is often herniation of the opposite lung across the midline into the involved hemithorax; in chronic atelectasis this may become extensive. When there is right-lower-lobe atelectasis, the height of the right hemidiaphragm may not be ascertained. There is usually enough gas in the stomach or colon to outline the left hemidiaphragm, however. The cause of the atelectasis may be evident on the film and may add to the opacity (Fig. 30-1).
Lobar Atelectasis
Lower-Lobe Atelectasis
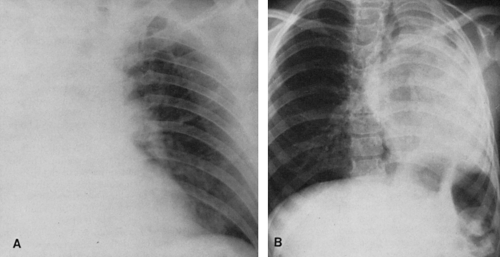
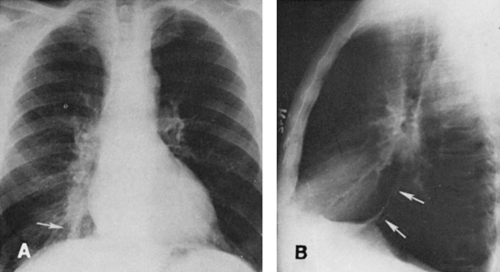
Lower-lobe atelectasis is easily overlooked, particularly on the left side, where the lobe may be hidden by the heart. The inferior pulmonary ligament attaches the medial aspect of the lower lobe to the mediastinum, so the lower lobe does not migrate upward toward the hilum. Also, the ligament keeps the base of a collapsed lower lobe in contact with the diaphragm. Occasionally, however, the inferior pulmonary ligament is incomplete, with no diaphragmatic attachment. In these cases, a paraspinal mass may be apparent on the posteroanterior (PA) projection, but the mass may not be visible on the lateral view, and the hemidiaphragm is not obscured posteriorly by the adjacent density usually present in lower-lobe atelectasis. This variation usually is present on the left side. When there are no pleural adhesions, the involved lobe moves medially to form ultimately a rather narrow triangle, with the apex at the level of the hilum and the base at the diaphragm. In the lateral projection the earliest sign of decrease in volume of a lower lobe is downward and posterior displacement of the major interlobar fissure. Later, as the amount of atelectasis increases, the opacity of the atelectatic lobe may obliterate the shadow of the posterior aspect of the diaphragm on the affected side. If the collapsed lobe is sufficiently dense (e.g., retained secretions, fluid), the lower aortic silhouette and the paraspinal line may be effaced on the PA view; on the lateral projection the density of the collapsed lobe adds to the density of the lower thoracic vertebrae, so that they become as dense or slightly more dense than the upper thoracic vertebrae. The signs of mediastinal shift—mdownward displacement of the ipsilateral hilum which may be hidden by the collapse, diaphragmatic elevation, and decrease in size of the bony thorax—mmay be present to varying degrees or may be absent. If they are absent, there is usually enough compensatory overinflation of the upper lobe on the left or of the upper and middle lobes on the right to alert the observer to the possibility of lobar collapse. When the roentgenogram is of sufficient penetration, the triangular shadow of the collapsed left lower lobe is usually outlined behind the heart on the frontal projection. Right-lower-lobe collapse usually causes a similar shadow at the right medial base which may obliterate the cardiophrenic angle. On the lateral view, the inferior vena cava may be obliterated and the density of the lobe may simulate fluid in the major fissure.
Kattan39 described upper mediastinal and aortic changes in lower-lobe atelectasis. In right-lower-lobe collapse, there may be an upper triangle sign. This is caused by a shift to the right of the anterior triangle consisting of the right and left anterior pleuromediastinal lines laterally and the clavicle above. The lines converge below as the anterior junction line. The triangle contains thymic, lymphoid, and areolar tissue and is not very dense. Although the triangle may shift to the left on left-lower-lobe collapse, it is hidden by the aorta.
In severe left-lower-lobe atelectasis, the heart shifts to the left and may rotate in a clockwise direction to such an extent that the appearance is that of a slight right-anterior-oblique rotation of the heart (flat waist sign).39 Obliteration of the top of the aortic knob (top-of-the-knob sign) may also occur in left-lower-lobe atelectasis. The lateral aspect of the left-upper mediastinum is continuous with the left border of the pericardium below the aortic knob.
On the right side, the vascular structures consisting of the right descending pulmonary artery, the superior pulmonary vein crossing it, the middle-lobe artery, and arteries to the superior segment of the lower lobe form distinct converging points at the hilum. The right-upper-lobe arteries form a less distinct converging point above this level. In the presence of atelectasis of the right upper lobe, there is only one converging point. The same is true of right-lower-lobe collapse. Therefore, when one vascular converging point is present on the right, lobar collapse is suggested. The sign is not pertinent on the left, where there is only one converging point.23
Right-middle-lobe Atelectasis
Right-middle-lobe atelectasis may be complete or incomplete. When this lobe decreases in volume, the secondary interlobar fissure moves downward; it and the primary fissure can be outlined in contrast to the dense lung between them on the lateral projection. The middle lobe also tends to move medially so that in the PA view a triangular shadow appears medially above the diaphragm. Its base is at the mediastinum, and the apex points toward the lateral chest wall. As atelectasis becomes complete, the lobe may shrink to a very small size and may be difficult to see clearly in the PA projection. The opacity caused by the atelectatic lobe blurs the right cardiac margin. When this blurring is noted, the lateral view is confirmatory. In the lateral view, varying degrees of collapse are readily outlined. The appearance is that of a dense triangle, the apex of which is at or near the hilum and the base of which points downward and anteriorly. The upper and lower borders of this dense triangle may be slightly concave.
Collapse of the lateral segment only does not cause blurring of the right cardiac margin. Usually there is no discernible mediastinal shift, hilar displacement, alteration of the right hemidiaphragm, or detectable compensatory overinflation of upper and lower lobes because of the small volume of the middle as compared to the upper and lower lobes.
Upper-lobe Atelectasis
In upper-lobe atelectasis the roentgen appearance depends on the presence or absence of adhesions between the visceral and parietal pleurae. When adhesions are present they may hold all or part of the lobe in its normal position; this peripheral upper-lobe collapse can simulate local pleural disease or loculated pleural effusion, particularly in infants. The
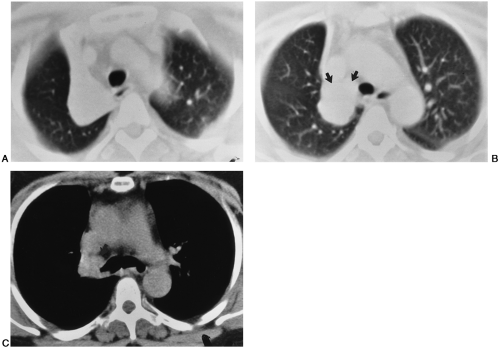
margins of the collapsed lobe are outlined by the overinflated lower lobe. When diagnosis becomes a problem, CT may be useful22 (Fig. 30-2). If there are no adhesions, the lobe tends to shrink uniformly and move toward the hilum.
margins of the collapsed lobe are outlined by the overinflated lower lobe. When diagnosis becomes a problem, CT may be useful22 (Fig. 30-2). If there are no adhesions, the lobe tends to shrink uniformly and move toward the hilum.
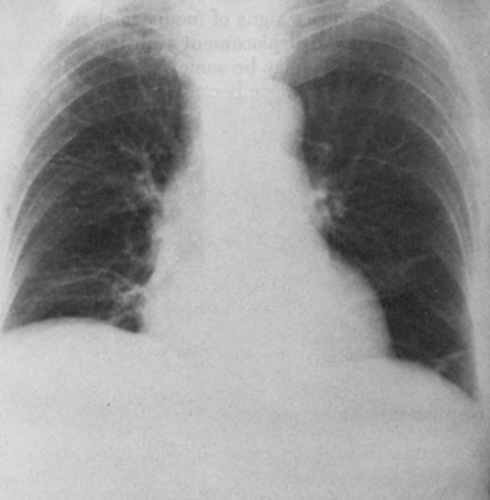
In right upper-lobe atelectasis, the first sign is elevation of the interlobar fissure. If there are lateral adhesions, the inferior aspect of the lobe becomes concave, with elevation of the minor fissure in its central aspect. There may be a slight increase in opacity of the lobe if fluid is present within it. With progressive collapse, the lobe shrinks and flattens against the apex and upper mediastinum. Partial adherence of the lobe in one area or another may alter the general contour, so a considerable variety of forms is possible. In the lateral view, the major fissure tends to move anteriorly with increasing atelectasis and is usually visible; the upward displacement of the minor fissure may also be outlined in this projection. However, the atelectatic upper lobe may be difficult or impossible to identify in the lateral view. The middle lobe moves upward anteriorly, and the lower lobe upward posteriorly. Its superior segment may actually occupy the apex and rotate forward to lie in a cap-like manner over the collapsed upper lobe. This can sometimes be outlined in the lateral projection and can be predicted by the appearance of normally aerated or hyperaerated lung at the apex above the more dense atelectatic upper lobe in the frontal projection. The signs of mediastinal and tracheal displacement toward the involved side, elevation of the right hemidiaphragm and hilum, and decrease in size of the hemithorax, resulting in narrowing of the intercostal spaces, may also occur in varying degrees, but compensatory hyperinflation of the middle and lower lobes may be sufficient so that none of the other signs is present. Mediastinal displacement may be a prominent feature when there are extensive adhesions holding the upper lobe to the lateral parietal pleura. The hilum may also be elevated and retracted to the ipsilateral side in such cases. When atelectasis is relatively complete and there are no adhesions, the shadow of the upper lobe tends to move toward the hilum and upper mediastinum. On the right side, it may then resemble slight mediastinal widening and occasionally may become so small that it is difficult to recognize.
Kattan and associates40 described a local elevation of the diaphragm, the juxtaphrenic peak, which is seen on the PA roentgenogram as a small shadow projecting upward from the highest point on the dome of the diaphragm. The shape varies from a narrow-based peak to a broad tent and is sometimes
more round than angular. A similar peak may be caused by an inferior accessory fissure or by tangential projection of the medial aspect of the major fissure, so differentiation must be aided by other signs of atelectasis in some patients. The cause is uncertain, but it may be that a small amount of basal pleura with or without a wedge of lung tissue is pulled upward by negative intrathoracic pressure that increases when lobar collapse occurs.
more round than angular. A similar peak may be caused by an inferior accessory fissure or by tangential projection of the medial aspect of the major fissure, so differentiation must be aided by other signs of atelectasis in some patients. The cause is uncertain, but it may be that a small amount of basal pleura with or without a wedge of lung tissue is pulled upward by negative intrathoracic pressure that increases when lobar collapse occurs.
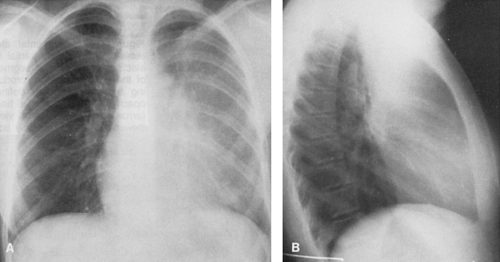
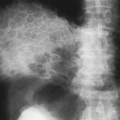
Atelectasis of the left upper lobe is somewhat similar to that of the right upper lobe, but the lobe moves superiorly, medially, and anteriorly rather than superiorly and anteriorly, and early change is more difficult to recognize in the frontal projection because there is no minor interlobar fissure. A ground-glass opacity is helpful if there is enough fluid in the collapsed lobe to produce it. In the lateral view the major fissure tends to move forward, and the opacity produced by the collapse is noted anteriorly with the lingula occupying a position similar to that of the middle lobe on the right. The partially collapsed lobe tends to be narrower inferiorly, owing to the smaller volume occupied by the lingula, and tends to extend upward and toward the periphery (Fig. 30-3). At times, the overinflated lower lobe may extend forward medial to a collapsed left lower lobe, where it produces a clearly defined area of lucency medial to the more opaque atelectatic upper lobe. The secondary signs of mediastinal shift-mhilar and diaphragmatic displacement and compensatory overinflation—may be somewhat greater than in right-upper-lobe collapse because of the difference in volume. The juxtaphrenic peak may be present. Volume loss produces shift of the mediastinum similar to that noted in right-upper-lobe disease, except that it is in the opposite direction. When collapse occurs in patients with chronic pulmonary inflammatory disease such as tuberculosis, there is often a considerable amount of associated pleural disease and contracting fibrosis of the lung. The lobe or segment involved is then difficult to evaluate on routine projections. In these instances, CT may be necessary for study of segmental anatomy (Fig. 30-4).
Segmental Atelectasis
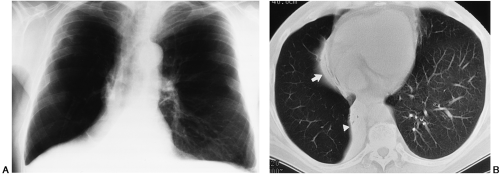
It is often possible to ascertain with a fair degree of accuracy the segment involved by segmental collapse, since the area of opacity produced by the atelectasis occupies the general area usually occupied by that segment in the absence of severely distorting associated pulmonary disease. By using frontal, lateral, and oblique projections as necessary, the site of the opacity can be established and its relation to the interlobar fissures ascertained. The fissure tends to bow toward the site of the atelectasis; for example, in anterior segmental atelectasis of the right upper lobe, the secondary interlobar fissure elevates centrally to indicate that the volume of this lobe has decreased, and in the lateral view the density lies anteriorly. The same rules apply for lower-lobe segments, but the basal segments are very difficult to identify accurately (Fig. 30-5). In questionable cases, CT may be helpful.
Focal (Plate or Lobular) Atelectasis
When there is obstruction of a small subsegmental bronchus, a small area of atelectasis may result. This produces a thin horizontal or plate-like line that is most often seen in the basal lung, where it occurs frequently. These small areas of atelectasis have been termed “plate,” “discoid,” “focal,” or “lobular” atelectasis. They vary in size and sometimes cannot be distinguished from small areas of fibrosis. In some instances, these linear opacities are caused by a combination of subpleural collapse of lung plus an invagination of overlying pleura. They tend to occur in areas of pleural clefts, indentations, scars, and incomplete fissures—all sites of pre-existing pleural invagination. Pulmonary embolism is present in many patients with focal atelectasis, but the areas of atelectasis do not represent infarcts or occluded vessels. It is likely that focal atelectasis often indicates more widespread peripheral atelectasis than is radiographically evident. Therefore, it may indicate a greater disturbance in ventilation than is apparent104 Films exposed at frequent intervals will show disappearance or change in position of the linear areas of density, and when this is evident the diagnosis of focal atelectasis is confirmed. The amount of involved lung is small, so the finding may be of little clinical significance, but, as indicated previously, it does indicate poor ventilation in the area. When it is observed postoperatively, it implies that aeration is incomplete and that there probably is an accumulation of secretions causing obstruction of some of the basal subsegmental bronchi. Restriction of diaphragmatic movement and elevation of the diaphragm are additional factors in production of this type of atelectasis (Fig. 30-6).
It is fundamental to remember that atelectasis causes an area of increased opacity because of a combination of airlessness or relative airlessness and some fluid in the involved
segment, lobe, or lung and that the resultant decrease in volume must be compensated by a decrease in total volume of the involved hemithorax or by an increase in volume of the uninvolved lobe or segments.
segment, lobe, or lung and that the resultant decrease in volume must be compensated by a decrease in total volume of the involved hemithorax or by an increase in volume of the uninvolved lobe or segments.
Chronic isolated atelectasis of the middle lobe has been described and termed middle-lobe syndrome. Although it is usually thought to be caused by inflammatory disease, several studies have indicated a high incidence of malignancy. In one study of 135 patients, 43% were found to have malignant tumors, so persistent atelectasis of the middle lobe is potentially caused by malignancy and should be managed as such.10
Round Atelectasis (Folded Lung)
Round atelectasis was originally described in Europe and more recently in the American literature.35 This lesion is formed in patients with pleural effusion, including those with asbestos-related pleural disease. A mass-like density is produced which must be differentiated from pleural and pulmonary tumors. The tumor-like mass is formed when a partially aerated portion of peripheral parenchyma floats on a pleural effusion and a collapsed section forms a groove, cleft, or fold on the lung surface. The floating lung tilts, and if this portion is lifted by the fluid, adhesions form. As the fluid recedes, a round mass of atelectatic lung is left adherent to the pleura. The adhesions cause the tilted lung to remain atelectatic. The normal lung then expands around the mass as the pleural disease regresses. At times, the round atelectasis retracts centrally and is surrounded by aerated lung. Some lesions persist; others gradually regress.
On plain films, there is evidence of pleural disease as well as a pleural-based round or oval mass ranging from 2.5 to 5 cm in diameter. A convergence of vessels and bronchi resembling a comet tail may be observed entering the mass. CT may be necessary to confirm the diagnosis.24,60 CT signs include the presence of a round or wedge-shaped peripheral mass abutting the pleura with adjacent pleural thickening and/or effusion. Bronchi and vessels are seen sweeping or curving into the mass (the “comet tail” or “vacuum cleaner” sign). Rounded atelectasis may show contrast enhancement as it represents rolled-up collapsed lung48,86,89,94,105 (see Fig. 27-11 in Chapter 27).
At times, the mass may be irregular, lobulated, and poorly defined, so it must be suspected in a patient with a juxtapleural mass with evidence of pleural disease.19
Computed Tomography in Lobar Collapse
When plain-film findings are atypical or equivocal, collapsed lung is associated with a pulmonary mass, fluid obscures the lung, or it is necessary to follow patients with lung tumor and atelectasis, CT may be of value for further evaluation.69,70,77 Lobar collapse causes compensatory overinflation, which usually can be identified on CT except in middle-lobe atelectasis, where volume loss is so small that there is little overinflation of the other lobes. Therefore, this is a finding common to collapse of all but the middle lobe.
In lower-lobe atelectasis (see Fig. 30-4), the lateral border of the collapsed lobe is concave or straight in the upper portion and either convex or concave inferiorly, so a convexity superiorly indicates an associated mass. The lobe collapses posteromedially and usually maintains contact with the diaphragm when the pulmonary ligament is complete. If the inferior pulmonary ligament is incomplete, the lobe may collapse toward the hilum. The major fissure is displaced medially, and the mediastinum is shifted toward the side of the lesion. Compensatory overinflation may be identified in contrast to the normal opposite lung. When pleural fluid is present, the collapsed lobe may be surrounded by the fluid, which may be less dense than the collapsed lobe.
When the right middle lobe (see Fig. 30-4) is collapsed, the configuration is triangular, with the apex directed laterally. The triangle is small at the hilar level and its inferior aspect. If the lobe is high (rotated or tipped upward), the volume of the triangle of density is increased. The triangle is decreased when the lobe is low (rotated inferiorly); because it is more vertical in position, it is seen on more CT planes than with the more horizontal position (rotated upward). Many variations in position of this lobe are possible when it is atelectatic, particularly if there is distortion secondary to adhesions.
The right upper lobe (see Fig. 30-2) collapses toward the mediastinum and may move toward the anterior chest wall and the lung apex to a varying extent. It appears as a mass in the anteromedial aspect of the upper thorax on CT. If
a tumor is present in the lobe, there is a convexity of the posterolateral aspect of the lobe; the size and location of the convexity are indicative of the size and location of the tumor. When the left upper lobe is collapsed, the lobe tends to move anteriorly and medially, and may move superiorly, so that it appears as an anteromedial density on CT. Pleural adhesions may alter the position of any collapsed lobe so that an atypical appearance is produced. When the upper lobe is collapsed peripherally in an anterior position, the superior segment of the lower lobe may move anteriorly between the upper lobe and mediastinum to produce a radiolucent triangle medial to the posterior aspect of the upper lobe. This radiolucency may appear on the PA film as a stripe between the aortic arch and the collapsed lobe. As a result, the aortic arch is very clearly defined. Lingular collapse alone is usually against the left heart border.
a tumor is present in the lobe, there is a convexity of the posterolateral aspect of the lobe; the size and location of the convexity are indicative of the size and location of the tumor. When the left upper lobe is collapsed, the lobe tends to move anteriorly and medially, and may move superiorly, so that it appears as an anteromedial density on CT. Pleural adhesions may alter the position of any collapsed lobe so that an atypical appearance is produced. When the upper lobe is collapsed peripherally in an anterior position, the superior segment of the lower lobe may move anteriorly between the upper lobe and mediastinum to produce a radiolucent triangle medial to the posterior aspect of the upper lobe. This radiolucency may appear on the PA film as a stripe between the aortic arch and the collapsed lobe. As a result, the aortic arch is very clearly defined. Lingular collapse alone is usually against the left heart border.
Endobronchial tumors can be identified within the lumen of a bronchus in most patients with obstructive atelectasis (see Fig. 30-2). The diagnosis of neoplasm is made more certain when a bolus of contrast material is used to aid in defining the tumor. Spiral CT scanning using narrow collimation and overlapping slice reconstruction obtained during a single breath hold optimizes CT detection of smaller endobronchial lesions. However, benign tumors cannot be differentiated from malignancies. Mucus plugs causing obstruction usually can be identified because of the branching pattern of the intrabronchial opacity, and the wall of the bronchus is visibly distinct from the mucus plug.
In nonobstructive atelectasis, CT is useful to demonstrate patency of bronchi when the bronchi are identified by careful evaluation of the bronchial tree in the involved area. Collapse secondary to pleural effusion can be seen, and any underlying pleural mass can be detected. In cicatrization atelectasis, the lung is often distorted; therefore, CT can provide valuable information regarding the location of the collapse, the absence of endobronchial mass, and the frequent association with bronchiectasis.
Atelectasis and Lung Torsion
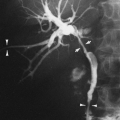
Atelectasis usually is present when there is torsion of the lung, a rare complication that occurs after traumatic pneumothorax or thoracic surgery. Occasionally it appears to occur spontaneously.28,64 The radiographic signs include (1) a collapsed or consolidated lobe in an unusual position; (2) hilar displacement in an inappropriate direction for the involved lobe; (3) alteration in normal position and sweep of pulmonary vessels; (4) rapid opacification of the involved lobe after trauma or surgery; (5) marked change (usually opacification) of the involved lobe on sequential films. When the condition is suspected on the chest radiograph, CT is usually indicated if there is any question of the diagnosis, because mortality is high if surgical fixation is delayed.
Pulmonary angiography may also be useful and may be diagnostic.66 Findings include slow filling and decrease in caliber of the arteries to the involved lobe and little filling of the veins except for possible retrograde filling of a short segment of the venous stump near the heart. The contrast agent tends to clear very slowly from the involved lobe.
When the middle lobe collapses after right upper lobectomy, findings may be similar to those of lobe torsion, so close clinical observation is necessary in these patients.88
ALTERED IMMUNITY AND THE LUNG
The immune system is composed of lymphocytes: the B (bursa) and T (thymic-derived) cells. The B cells secrete immunoglobulins and are responsible for humoral immunity. The immunoglobulins are IgG, IgA, IgM, IgE, and IgD. The T lymphocyte or T cell is responsible for cellular immunity. The immune responses are classified into four types: Type I is the IgE-dependent response, in which the antibody is E. There is an immediate skin test; the clinical examples are extrinsic asthma and anaphylaxis. Type II involves tissue-specific antibody; the antibodies are G and M. A clinical example is Goodpasture’s syndrome. Type III involves immune complexes. Antibodies are G and M. Clinical examples are extrinsic alveolitis produced by organic and inorganic dust and intrinsic alveolitis including collagen-vascular diseases and fibrosing alveolitis. The first three are examples of humoral immune responses. Type IV is the cell-mediated response (delayed hypersensitivity). Clinical examples are intracellular infections, graft rejection, and cancer suppression.80 Some of the conditions mentioned here are described in greater detail in the sections that follow.
Pulmonary Hemorrhage
Pulmonary hemorrhage can be categorized as focal or diffuse. Causes of focal pulmonary hemorrhage are infections (including tuberculosis), bronchiectasis, chronic bronchitis, and tumors. Patients with focal pulmonary hemorrhage usually (although not invariably) present with hemoptysis, and the chest film is unremarkable or nondiagnostic in 40%. When abnormalities are seen, they include focal air-space opacity or consolidation, atelectasis, and tumor mass or cavity.76 Further evaluation with CT examination is often indicated in occult cases and may reveal unsuspected bronchiectasis, tuberculosis, or an endobronchial lesion. Frequently, however, plain film roentgenography, CT, and bronchoscopy fail to determine the cause of the hemoptysis.
Diffuse pulmonary hemorrhage is characterized typically by bilateral air-space disease that often has a ground-glass appearance on chest films and CT (Fig. 30-7). Patients present with hemoptysis and dyspnea and may have iron deficiency anemia if episodes are recurrent. Bronchoscopy with bronchoalveolar lavage reveals the evidence of both recent hemorrhage in the air spaces and an abundance of hemosiderin-laden macrophages in the air spaces and in the interstitium.76 Causes of diffuse pulmonary hemorrhage include Goodpasture’s disease (antiglomerular basement membrane disease); collagen vascular or autoimmune disorders, such
as, systemic lupus erythematosus (SLE) or Wegener’s granulomatosis; idiopathic pulmonary hemosiderosis; bleeding disorders; drug reactions; infections; and tumors.76,107
as, systemic lupus erythematosus (SLE) or Wegener’s granulomatosis; idiopathic pulmonary hemosiderosis; bleeding disorders; drug reactions; infections; and tumors.76,107
Goodpasture’s Syndrome
Goodpasture’s syndrome is an autoimmune disease in which circulating antibodies have been demonstrated against the alveolar basement membrane and against glomerular basement membrane. In contrast to idiopathic pulmonary hemosiderosis, it occurs in young adults with a male predominance of 7:1 or 8:1.
Goodpasture’s syndrome is characterized by episodes of diffuse pulmonary hemorrhage, renal glomerulonephritis, and circulating antiglomerular basement membrane antibodies. Classic chest film and CT findings include bilateral, perihilar air-space consolidation or ground-glass opacity that spares the apices and costophrenic angles. As is typical for pulmonary hemorrhage from any cause, the air-space disease clears rapidly in a few days but then may give way to a reticular pattern of linear opacities and thickening of the interlobular septa as the acute hemorrhage clears. Atypical presentations including asymmetrical air-space disease and normal chest radiographs have been reported. The diagnosis should be suspected when a combination of hemoptysis and renal disease occurs in a young man. The diagnosis is confirmed by identifying the antibodies in the serum or appropriate immunofluorescent staining of the basement membrane on renal biopsy. Treatment is with high-dose corticosteroids and plasmapheresis.76
IDIOPATHIC PULMONARY HEMOSIDEROSIS
Hemosiderosis is a term used to indicate the presence of macrophages filled with hemosiderin deposited in the alveoli and interstitial tissues of the lung. The cause is unknown, but some immunologic mechanism is a distinct possibility. There is an injury at the level of the alveolarcapillary membrane that allows the leakage of blood into the interstitium and ultimately into the alveolar space of the lung. This is manifested by recurrent episodes of acute illness in which
dyspnea, cyanosis, and weakness along with cough, hemoptysis, and chest pain occur. The attack lasts a few days or weeks before subsiding. Recurrent episodes of bleeding ultimately produce marked roentgenographic changes. The prognosis is generally poor, although the disease may progress very slowly in some patients. It is somewhat more common in children than in adults. In children there is no sex predominance, but when it develops in adults, there is a preponderance of men of about 2:1 or 3:1.
dyspnea, cyanosis, and weakness along with cough, hemoptysis, and chest pain occur. The attack lasts a few days or weeks before subsiding. Recurrent episodes of bleeding ultimately produce marked roentgenographic changes. The prognosis is generally poor, although the disease may progress very slowly in some patients. It is somewhat more common in children than in adults. In children there is no sex predominance, but when it develops in adults, there is a preponderance of men of about 2:1 or 3:1.
The roentgen findings in the acute phase are those of alveolar hemorrhage, which causes widespread, patchy alveolar opacities that clear gradually over a period of days. Residual deposition of hemosiderin in the interstitium produces thickening that appears roentgenographically as an increase in interstitial markings, producing a reticular or reticulonodular appearance (Fig. 30-8). The diagnosis is made by correlating the clinical history with the roentgenographic changes. MRI may be of value83 in demonstrating the presence of widespread deposits of ferric iron throughout the lung. This may obviate biopsy or other invasive diagnostic methods, particularly in a critically ill patient. An association with celiac disease has been reported, but a pathogenetic link between the two diseases has not been established.73 The disease must be differentiated from a number of other diseases that cause widespread interstitial change of this type and from other diseases that cause lung hemorrhage.
Pulmonary Changes after Hemoptysis
When hemoptysis occurs and blood is aspirated, the roentgen findings vary with the amount and distribution of the blood. An opacity comparable to that of a patch of pneumonia of similar size is produced. It is usually hazy and poorly defined and may be local or widely scattered, depending on the sites and amount of bleeding. The opacity usually clears within 2 or 3 days, which aids in differentiating hemorrhage from inflammatory disease. Evidence of the disease causing the hemoptysis may or may not be present. In patients with pulmonary contusion causing hemorrhage, a relatively clearly defined mass (hematoma) may appear which is unlike the more diffuse change usually observed in patients with hemoptysis. It changes more slowly.
EOSINOPHILIC LUNG DISEASE—mPULMONARY INFILTRATES WITH EOSINOPHILIA (PIE)
Eosinophilic disease includes a group of widely varied disorders. Several classifications have been used, none of which is entirely satisfactory because of the number of etiologic factors, the diversity of clinical syndromes, and the fact that tissue eosinophilia occurs in some patients, blood eosinophilia in others, and both tissue and blood eosinophilia in still others.20A One of the classifications is termed the PIE syndrome. There are five major categories: (1) Löffler’s syndrome; (2) chronic eosinophilic pneumonia or a chronic form of pulmonary opacities with eosinophilia; (3) chronic PIE with asthma; (4) tropical eosinophilia; and (5) polyarteritis or vasculitis in association with pulmonary opacities, which are the predominant part of the disease. There may be many manifestations in other organ systems in polyarteritis.52
Löffler’s Syndrome (Transient Pulmonary Infiltration with Eosinophilia, Acute Eosinophilic Pneumonia)
Löffler’s syndrome consists of fleeting pulmonary opacities associated with eosinophilia. Usually the subjects are allergic individuals, and the disease is believed to represent a pulmonary reaction to a variety of allergens. The symptoms are usually mild and consist of cough, malaise, low-grade fever, dyspnea with occasional wheezing, mild chest pain, and metallic taste in the mouth. It is associated with eosinophilia that ranges from 10% to 70%, and usually also with leukocytosis. The amount of pathologic material available is small because this is a relatively benign condition, but the findings in patients who have died accidentally consist of eosinophilic pneumonia in which the involvement is interstitial as well as alveolar. There is also some associated pulmonary edema, probably secondary to increased permeability of capillaries. Acute eosinophilic pneumonia can cause noninfectious respiratory failure, however. Although the symptoms may be severe, the disease usually responds quickly to corticosteroids.4
Roentgen Findings
The disease produces poorly defined opacities that may be single or multiple, unilateral or bilateral. The volume of lung affected varies considerably, and the individual areas of involvement are patchy in type and usually poorly outlined. They resemble pneumonia of other causes but are unique in that the homogenous densities are usually peripheral and rapid change is the rule. It is not unusual to observe clearing or partial clearing in one area and progression in another area of the same lung or of the opposite lung. In severe cases, pulmonary consolidation may be extensive and bilateral. Without therapy, change may be very slow. At times, the findings remain stable for several days. When such a changing opacity is observed and the patient is found to have eosinophilia, the diagnosis of Löffler’s syndrome can be made (Fig. 30-9). A minor amount of pleural reaction resulting in small amounts of pleural effusion may occur, but the presence or absence of effusion is of no diagnostic significance.
Because similar eosinophilic pneumonias may be caused by a variety of parasitic infestations, by infections, and by drugs, the term eosinophilic pneumonia should be used to denote those of unknown cause. The others are better designated as eosinophilic pneumonia caused by a specific drug, infestation, or other cause. The administration of cortisone usually produces rapid clearing of the pulmonary disease and a decrease in the circulating eosinophils.
Chronic Eosinophilic Pneumonia
Chronic eosinophilic pneumonia is similar to Löffler’s syndrome except that the symptoms are prolonged and the course is more malignant. The disease usually occurs in women. Its onset may be sudden and its duration may extend from months to years. Episodes of weakness, weight loss, fever, cough, dyspnea, and hemoptysis may occur. Roentgen findings consist of a variety of patterns of opacity, some resembling confluent pneumonia, others consisting of
coarse, strand-like densities that may be widespread. Although the disease tends to be peripheral in the upper lung zones, it may or may not appear so on plain films (Fig. 30-10). CT is more likely to identify the peripheral location of the pulmonary consolidation when the location is not certain in patients suspected of having chronic eosinophilic pneumonia. Mediastinal adenopathy has also been reported on CT studies.58 Changes in the pulmonary disease are common, as in Löffler’s syndrome. Eosinophils are found in the biopsy specimens, which show interstitial pneumonia of varying degrees of severity, sometimes associated with fibrosis. There is also an elevated eosinophil count in the circulating blood. The lesions do not respond to antibiotics but clear dramatically with steroid therapy.
coarse, strand-like densities that may be widespread. Although the disease tends to be peripheral in the upper lung zones, it may or may not appear so on plain films (Fig. 30-10). CT is more likely to identify the peripheral location of the pulmonary consolidation when the location is not certain in patients suspected of having chronic eosinophilic pneumonia. Mediastinal adenopathy has also been reported on CT studies.58 Changes in the pulmonary disease are common, as in Löffler’s syndrome. Eosinophils are found in the biopsy specimens, which show interstitial pneumonia of varying degrees of severity, sometimes associated with fibrosis. There is also an elevated eosinophil count in the circulating blood. The lesions do not respond to antibiotics but clear dramatically with steroid therapy.
CHRONIC INTERSTITIAL PNEUMONIAS (DIFFUSE FIBROSING ALVEOLITIS)
The term chronic interstitial pneumonia, as used by Liebow and Carrington,53 indicates a pneumonia in which the most significant or persistent component of the tissue response in the lung is in the interalveolar septa and more proximal supporting tissues. Others, including Scadding and Hinson,84,85 prefer the term fibrosing alveolitis to describe these diseases, many of which are of unknown cause. They believe that the categories described by Liebow and Carrington are not very clear, so the entire group of chronic interstitial pneumonias (fibrosing alveolitis) remain somewhat controversial. They then restrict the term “pneumonia” to denote inflammation of the lung characterized by consolidation produced by exudates filling the alveoli. Some believe the group of idiopathic interstitial pneumonias represent inflammatory responses of the alveolar walls to injuries of different types, durations, and intensities and are thus different aspects of a multifaceted “fibrosing alveolitis.”
In view of the lack of knowledge regarding etiologic factors, two partial classifications are necessary—mone histopathologic, the other etiologic. They are not mutually exclusive, because the cause is not often determined. The chest film findings in chronic interstitial lung disease progress at
varying rates. The earliest manifestation is a fine nodularity, which is followed by or associated with a fine reticular or reticulonodular pattern. This may change to a coarse reticular or reticulonodular pattern with cystic areas. The most chronic form of the interstitial pneumonias is represented by interstitial fibrosis. In this end stage there is usually breakdown of alveolar walls in addition to fibrosis, which produces the so-called honeycomb pattern of pulmonary interstitial fibrosis. The honeycomb pattern usually implies far-advanced disease in that part of the lung, but not necessarily in all of the lung. Since the chest film findings are not a sensitive indicator of progression of interstitial lung disease; high-resolution computed tomography (HRCT), biopsy, and/or examination of cells obtained by bronchoalveolar lavage as well as gallium scans are used for this purpose. About 30% of patients with biopsy-proved alveolitis have normal chest roentgenograms.
varying rates. The earliest manifestation is a fine nodularity, which is followed by or associated with a fine reticular or reticulonodular pattern. This may change to a coarse reticular or reticulonodular pattern with cystic areas. The most chronic form of the interstitial pneumonias is represented by interstitial fibrosis. In this end stage there is usually breakdown of alveolar walls in addition to fibrosis, which produces the so-called honeycomb pattern of pulmonary interstitial fibrosis. The honeycomb pattern usually implies far-advanced disease in that part of the lung, but not necessarily in all of the lung. Since the chest film findings are not a sensitive indicator of progression of interstitial lung disease; high-resolution computed tomography (HRCT), biopsy, and/or examination of cells obtained by bronchoalveolar lavage as well as gallium scans are used for this purpose. About 30% of patients with biopsy-proved alveolitis have normal chest roentgenograms.
The radiologist does have a role in the study of the interstitial lung disease and should evaluate the following: (1) distribution of disease and lung volume; (2) progression of the opacities; (3) presence of hilar nodes; (4) pleural abnormalities; (5) presence of conglomerate masses; and (6) malignant change. (There is an increased relative risk for development of lung cancer of 14.1 in patients with idiopathic pulmonary fibrosis.41,50) A high degree of radiographic diagnostic accuracy is not possible, but accuracy can be increased when the chest film is read in conjunction with an HRCT scan.100
On the basis of histologic criteria and to some extent on radiographic and clinical criteria, Liebow and Carrington53 described the following interstitial pneumonias:
UIP—the classic, “undifferentiated” or “usual” interstitial pneumonia (Figs. 30-11 and 30-12)
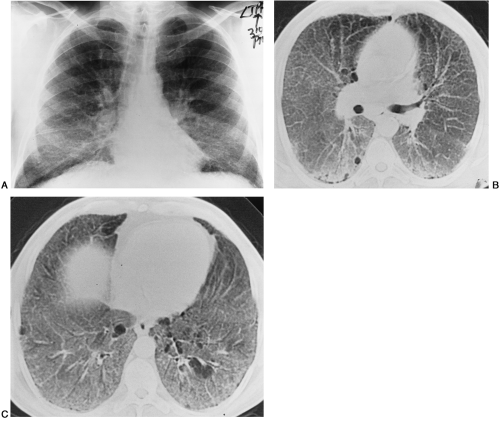
BIP—nonbacterial bronchiolitis obliterans superimposed on UIP, now referred to as bronchiolitis obliterans organizing pneumonia (BOOP) or cryptogenic organizing pneumonia (COP) (Fig. 30-13)
DIP—desquamative interstitial pneumonia
LIP—lymphoid interstitial pneumonia (Fig. 30-14)
GIP—giant-cell interstitial pneumonia.
GIP is now thought to be caused by hard-metal pneumoconiosis. LIP is recognized as a lymphoproliferative disorder rather than a true interstitial pneumonia. Many authorities no longer consider UIP and DIP to be separate diseases, but different stages of the same process of idiopathic pulmonary fibrosis. For an in-depth discussion, the reader is referred to an excellent review of the chronic interstitial pneumonias by McAdams and associates.59
As noted in the following descriptions, the interstitial pneumonias exhibit a rather wide variety of roentgenographic patterns. The use of CT, including thin-section HRCT, in the study of patients with the various forms of chronic interstitial pneumonias including end-stage disease has been studied by many. These techniques are being used increasingly in patient management and diagnosis. Even with lung biopsy and histologic examination of tissue, the cause is not clear in most instances. As indicated previously, many prefer the term fibrosing alveolitis for these conditions in which there is ultimately more or less interstitial fibrosis.
Classic or Undifferentiated Interstitial Pneumonia (UIP)
This disease is the result of diffuse alveolar damage from a large variety of agents, including a number of inhalants such as oxygen in high concentrations, particularly when administered by intermittent positive-pressure breathing machines;
viruses and mycoplasma; and conditions with altered immunity, such as scleroderma and rheumatoid arthritis. A number of drugs, bleomycin in particular, also can cause fibrosing alveolitis. In some cases a genetic factor may be involved. However, the cause of most chronic interstitial pneumonias is unknown. Whatever the cause, diffuse alveolar damage is produced, probably with some necrosis of alveolar epithelium and proteinacious exudates. The basement membrane usually is preserved. Hyaline membranes comprising exudate and remnants of necrotic alveolar lining cells may be present. Interstitial infiltrations of lymphocytes and mononuclear cells are also noted in this stage. Interstitial proliferation and fibrosis eventually occur in some areas. During the acute phase, the exudates plus the swelling of alveolar cells and the interstitial infiltrate produce a roentgen picture simulating the hazy appearance of pulmonary edema. Roentgenographic findings vary considerably. In the early fibrotic phase, a fine reticular pattern may be observed, often predominantly basal; in the more chronic forms of UIP, the opacity produced is coarse and strand-like and tends to extend radially from the hilum. Small, round radiolucencies may appear and become more prominent with increasing fibrosis and destruction of the alveolar walls; this represents the honeycomb pattern of pulmonary fibrosis. There is often an associated loss of lung volume, which suggests either UIP or scleroderma. As mentioned, radiographic findings are not specific.
viruses and mycoplasma; and conditions with altered immunity, such as scleroderma and rheumatoid arthritis. A number of drugs, bleomycin in particular, also can cause fibrosing alveolitis. In some cases a genetic factor may be involved. However, the cause of most chronic interstitial pneumonias is unknown. Whatever the cause, diffuse alveolar damage is produced, probably with some necrosis of alveolar epithelium and proteinacious exudates. The basement membrane usually is preserved. Hyaline membranes comprising exudate and remnants of necrotic alveolar lining cells may be present. Interstitial infiltrations of lymphocytes and mononuclear cells are also noted in this stage. Interstitial proliferation and fibrosis eventually occur in some areas. During the acute phase, the exudates plus the swelling of alveolar cells and the interstitial infiltrate produce a roentgen picture simulating the hazy appearance of pulmonary edema. Roentgenographic findings vary considerably. In the early fibrotic phase, a fine reticular pattern may be observed, often predominantly basal; in the more chronic forms of UIP, the opacity produced is coarse and strand-like and tends to extend radially from the hilum. Small, round radiolucencies may appear and become more prominent with increasing fibrosis and destruction of the alveolar walls; this represents the honeycomb pattern of pulmonary fibrosis. There is often an associated loss of lung volume, which suggests either UIP or scleroderma. As mentioned, radiographic findings are not specific.
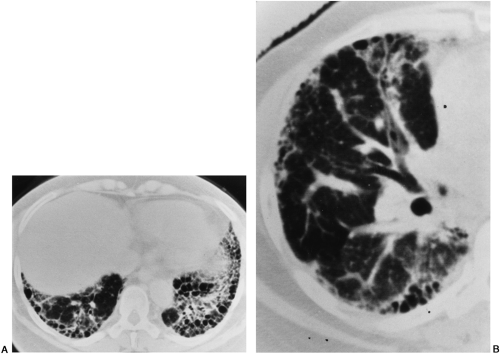
HRCT findings of UIP, fibrosing alveolitis, or idiopathic pulmonary fibrosis include reticulation, fibrotic thickening of the interlobular septa, architectural distortion, honeycombing, and traction bronchiectasis (see Figs. 30-11 and 30-12).100 The changes are more prominent in the peripheral subpleural zone, posteriorly, and at the lung bases. Varying degrees of ground-glass opacity may be seen in UIP; this may be caused by alveolitis in some patients, but in others biopsy shows only early alveolar wall interstitial thickening.
Ground-glass opacity may be associated with areas of fibrosis and honeycombing. In other patients, garlands or arcades of ground-glass opacity outline the subpleural zones of the lung with increased lung attenuation. Although ground-glass opacity may be diffuse and the predominate feature in DIP, it is rarely so in idiopathic pulmonary fibrosis (IPF). More often it is a secondary feature to findings of fibrosis. As in all causes of interstitial lung disease, a prominent feature is the irregular interface sign: wherever the lung interfaces with another structure, be it the mediastinum, the pleura, the fissures, or the pulmonary bronchovascular bundles, the interface formed with the lung is shaggy and irregular. As pulmonary fibrosis advances to end-stage lung disease, honeycombing and subpleural cysts become the predominant feature.
Ground-glass opacity may be associated with areas of fibrosis and honeycombing. In other patients, garlands or arcades of ground-glass opacity outline the subpleural zones of the lung with increased lung attenuation. Although ground-glass opacity may be diffuse and the predominate feature in DIP, it is rarely so in idiopathic pulmonary fibrosis (IPF). More often it is a secondary feature to findings of fibrosis. As in all causes of interstitial lung disease, a prominent feature is the irregular interface sign: wherever the lung interfaces with another structure, be it the mediastinum, the pleura, the fissures, or the pulmonary bronchovascular bundles, the interface formed with the lung is shaggy and irregular. As pulmonary fibrosis advances to end-stage lung disease, honeycombing and subpleural cysts become the predominant feature.
Diffuse Alveolar Damage and Bronchiolitis Obliterans (BIP)
When there is damage to bronchioles superimposed on the lesion of UIP, BIP is produced; as mentioned, this is now referred to by many as BOOP or COP (Fig. 30-13).
Although the cause is not clear, this kind of damage can result from inhalation of corrosive fumes of strong acids. However, it appears to occur much more commonly as a result of a necrotizing bacterial bronchiolitis superimposed on viral pneumonia. The radiographic findings consist of streaks of flame-like opacity noted chiefly in the upper and central lung, although they can occur anywhere in the lungs. This may represent UIP with superimposed bacterial infection rather than a distinct entity.
Desquamative Interstitial Pneumonia (DIP)
DIP is an interstitial pneumonia characterized by extensive proliferation and desquamation of granular pneumocytes (type II alveolar-lining cells). It is associated with a mild interstitial cellular infiltration of plasma cells, lymphocytes, and eosinophils and with some septal and pleural edema. These desquamated cells and large aggregated macrophages may fill the bronchioles as well as alveoli. As in the other diseases affecting the alveolar walls, the cause is not certain, but in many an immunologic mechanism is a factor.
Roentgenographic findings consist of bilateral basal shadows which often are hazy and may have a ground-glass appearance. As the disease progresses, the basal density increases. The pattern is variable, however, with more density in upper than in lower lungs in some patients. In many of our patients, the disease has been widely scattered with pulmonary opacities of varying configuration, often with a mixed interstitial and alveolar pattern (Fig. 30-14). We have observed very few patients with “classic” medial-basal “ground-glass” disease as originally described by Liebow.53 There is usually a favorable response to steroids; occasionally, however, the disease may progress to a nonspecific, honeycomb pattern of fibrosis with loss of pulmonary volume (the end-stage lung).27,32 On HRCT, ground-glass opacities tend to be more diffuse and the predominant feature in many cases of DIP.
Lymphoid Interstitial Pneumonia (LIP)
In LIP there is massive and widespread infiltration of both lungs by lymphoid tissue, which histologically resembles lymphoma very closely. The history is that of a very chronic interstitial pneumonia with cough, dyspnea, fever, and weight loss over a long period. The infiltration is interstitial in the interalveolar septa and in the peribronchiolar and perivenous spaces. Local lymph nodes and extrapulmonary tissues are not involved. The infiltrate is a mixture of small lymphocytes, plasma cells, and occasionally large mononuclear cells, with the small lymphocytes predominating. Although the cause is not certain, some type of hypersensitivity appears to be a factor in most patients. Radiographic changes are variable and range from bilateral diffuse nodular opacities to peripheral opacities, which may be linear or branching,




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree