Pain is the unpleasant sensory experience following tissue damage or the threat of damage. The activation of cortical regions during noxious stimulation is believed a result of the negative affect and sensations generated by the stimulus. How a noxious event is translated into pain experience remains uncertain, and pain that occurs in the absence of a noxious event remains mysterious. Acute pain and chronic pain depend on a categorization of feeling that occurs collectively rather than individually. Capturing that process inside a brain scan is problematic. Resolving this problem requires an approach to imaging that transgresses the boundaries of physical and social concepts.
Almost 350 years ago, Descartes proposed that noxious stimuli transfer energy to “threads” running through the body to open pores in the brain and signal pain . Pain thus was produced just as pulling on a rope might cause a bell to ring. Descartes was proposing a theory of pain based on the specificity of the stimulus and a dedicated processing system designed to receive specific stimulus information. In the modern interpretation, pain fibers, rather than threads, are stimulated and relay information to a hypothesized pain center somewhere in the brain.
Descartes theory was purposely mechanistic with respect to the function of the body, which he believed could be understood using mechanical models and contemporary scientific insights. In 1637, for example, Descartes reported an experiment in which he scraped the tissue from the back of an ox’s eye and placed the eye in a shutter, allowing light to enter the front of the eye . Descartes reported that he saw an inverted and reversed image on a sheet of thin paper placed where the retina would have been in the living animal, demonstrating that the eye was a kind of camera obscura. Thus, Descartes demonstrated the mechanical or physical beginnings of vision, but he did not accept that vision was merely was the consequence of physical action in the eye and brain. In the Second Meditation , Descartes explained, “perception is neither an act of vision, nor of touch… but only an intuition of the mind” . The orderly and calculable penetration of light rays through the camera obscura exposes the light to the reason of the mind. The mind is not dazzled by the senses.
Notoriously, Descartes separated the mechanical function of the body from reasoning and placed the former into the hands of a burgeoning scientific exploration and the latter into the hands of the church. This is misunderstood widely as a concession brought about by Descartes’ reluctance to challenge the authority of the church but the truth is more complex. Descartes had a deep regard for human reason, viewing it as the source of free will, and recognized that mechanical interpretations of human beings undermined reason and free will . Beginning with the certainty of first-person cognition, Descartes set out to prove the existence of God and thus reconcile the apparent mechanical action of the body with the existence of reason via an interaction of the physical with spiritual omnipotence. In today’s more secular and frankly misanthropic age, God has been ejected and reason minimized . In consequence, there is an unwitting return to Descartes’ mechanical vision of human beings without any obvious means to reconcile the mechanics of the human body with the existence of human experience. This schism in the current philosophy of mind is problematic particularly for neuroscientific investigations of pain, because it feels like something to be in pain, and that something is not captured directly by the physical events in the body and brain .
What is pain?
During World War II, Henry Beecher observed that only one out of three soldiers wounded in battle complained of sufficient pain to receive morphine . Most soldiers denied having any pain or claimed so little as to not require pain relief. A 1978 study of Israeli soldiers who had traumatic amputations after the Yom Kippur War replicated Beecher’s report , and a 1982 study demonstrated similar findings in a civilian setting . Approximately 40% of patients presenting to the emergency department of a London hospital complained of less pain than might be expected based on the extent of their injuries.
Patients who have injuries but no pain undermine the linkage of a single, specific, psychologic experience with a noxious stimulus. Consequently, theories of pain based on a line system that is activated by noxious information and dedicated to generating an experience of pain gradually have given way to a more distributed understanding of pain physiology and broader conceptions of pain experience. In 1965, Melzack and Wall proposed a new theory of pain processing, known as the gate control theory . The theory proposed that pain is the result of the relative activity in small and large diameter nerve fibers. Large diameter fibers carry mechanical sensory information; small diameter fibers carry noxious information. Small fiber activity tends to facilitate the passage of information up the spinal cord (opening the gate), whereas large fiber activity inhibits the flow of information (closing the gate). Melzack and Wall reasoned that the interneuronal cells of the spinal cord must select and compute outputs based on the combination of signals received. A descending influence on these cells from the brain also was included, which in turn was influenced by input from the spinal cord, thus forming a spinal cord-brain loop. Gate control theory provided the first physiologic mechanism for psychologic interventions to minimize pain, such as distraction or relaxation, and shifted attention away from the peripheral source of injury and toward the spinal cord and brain.
As a reaction against the failures of specificity and in an effort to incorporate new facts about the biologic system for processing noxious information, many theories have followed that can be characterized broadly as “pattern theories.” The most popular of these is the biopsychosocial model of pain . The biopsychosocial approach to pain is based on several propositions, the central one being that individuals’ emotions and behavioral activity in response to an event are influenced by their appraisal of that event and environmental circumstances. In addition to the biology of a noxious event, the biopsychosocial model introduces psychologic and social factors that may mitigate or enhance the final experience of pain. Crucially, the biopsychosocial model places an emphasis on the content of pain experience rather than on the source of noxious information .
Emphasis on the content of pain also characterizes the International Association for the Study of Pain (IASP) definition of pain. The IASP defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” . By including the content of pain, the IASP definition avoids becoming circular—defining pain as the response to pain .
Pain no longer is regarded merely as a physical sensation of noxious stimulus and disease but is seen as a conscious experience that may be modulated by mental, emotional, and sensory mechanisms and includes sensory and emotional components. The definition of pain states further, “…pain is always subjective. Each individual learns the application of the word through experiences related to injury in early life.” This definition encourages an understanding of pain as an emergent property of social awareness rather than as a deterministic or spiritual phenomenon.
The IASP definition of pain and the biopsychosocial concept can account for the variable relationship between pain and injury and the multidimensional nature of pain. The definition and the biopsychosocial model also predict that pain involves many distributed regions of the brain, which has been demonstrated by functional imaging studies.
Functional imaging during noxious stimulation
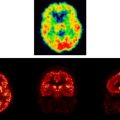
Many studies have examined the brain response to acute pain in healthy subjects using positron emission tomography, functional MRI, magnetoencephalography, and electroencephalography and are reviewed extensively elsewhere . Similar techniques also have been extended to the study of chronic pain disorders, such as irritable bowel syndrome, fibromyalgia, atypical facial pain, and nonspecific low back pain. The persistence, intractability, and apparent absence of peripheral disease to account for these so-called “functional” pain disorders has led to particular interest in the possibility of a central etiology and the use of functional imaging to test central hypotheses. Figs. 1 and 2 summarize the responses observed in healthy controls and patients who have functional pain on the lateral (see Fig. 1 ) and medial (see Fig. 2 ) surfaces of the brain.
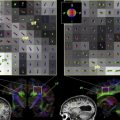
Fig. 1 provides a lateral surface illustration of the brain response to pain for studies involving noxious phasic somatic stimuli (ie, continuously alternating intensity or switching location), tonic somatic stimuli (constant in position and intensity), and visceral stimuli. Each circle represents the reported coordinates from various pain studies (reviewed and referenced elsewhere) . There are more studies reported of somatic than of visceral pain, thus, the actual number of circles is incidental to any pattern differences that can be observed. The actual number of circles is, therefore, incidental to any pattern differences that can be observed.
There is clear contralateral clustering of primary sensory activation during somatic sensation. Variability in the precise position of S1 activation has been noted previously and discussed as a possible consequence of the lack of spatial resolution . There is a large cluster, including secondary somatosensory response (S2, in and around Brodmann’s area [BA] 42/43) and an area anterior to S2, that corresponds largely to the facial motor region, perhaps indicating grimacing or verbalization or possibly the surface projection of anterior insula. In addition, there is a large cluster of activation, posterior and superior to S2, around the inferior parietal region (BA 40 encroaching onto BA 39). Further clustering is apparent anterior to the motor region in BA 45 and 46 of the prefrontal cortex and an overall dominant lateralization of prefrontal response to the right hemisphere regardless of the side of stimulation.
A similar pattern of clustered activation is apparent during visceral distension of the upper and lower gastrointestinal tract, especially in and around S2 and in the right prefrontal cortex. The diffuse and poorly localized nature of visceral pain suggests that S1 activation should be less apparent and this is confirmed partially in Fig. 1 . Even within S1, however, there are overlapping clusters of activation when comparing somatic with visceral. Studies of patients who have somatic and visceral functional pain also show activation of S1.
Fig. 2 shows the medial surface clustering for the same studies as in Fig. 1 . Somatic pain provides for common activation of the midcingulate region extending into motor regions and for the thalamus and distinct activation of the cerebellum. Visceral distension also provides for extensive activation of the midcingulate region extending into motor regions. Patients who have functional pain activate a similar spread of midcingulate regions.
In summary, a variety of functional imaging studies of pain demonstrate common activation of primary and secondary somatosensory, prefrontal, and midanterior cingulate cortices. These cortical regions have been demonstrated to activate in proportion to the intensity of pain experience and decrease activation in proportion to pain reduction via drug anesthesia , drug analgesia , placebo , or other pain reduction techniques . The pattern of activation observed during pain experience across controls and patients who have functional disorder is remarkably consistent when considering the variable techniques of measurement and noxious stimulation.
Melzack has proposed that the brain response to pain be conceptualized as a “neuromatrix”—a network of neurons responding to noxious information and mediating pain experience. To incorporate the sensory, affective, motoric, and cognitive elements that are part of the pain experience, Melzack proposed an extensive matrix, including at least three major neural circuits in the brain. One is the classical sensory pathway; a second pathway is through the brain stem to the limbic system; and a third is to parietal association regions. These neural circuits also can be divided according to the medial and lateral pain systems . Definition of the lateral and medial pain systems depends on the divergence of spinothalamic projections in the thalamus. In terms of the lateral pain system, spinothalamic projections largely are to the ventral posterior lateral and ventral posterior inferior nuclei, which project in turn to primary and secondary somatosensory cortices. These pathways transmit information regarding the intensity, duration, and location of noxious stimuli with few alterations in coding parameters between the spinal cord and cortex and, thus, are ideal for providing information about the location and characteristics of a noxious stimulus.
The medial pain system comprises spinothalamic projections to medial thalamic nuclei and from there to limbic cortices, including the anterior cingulate cortex (ACC). In addition, the periaqueductal gray (PAG) has reciprocal connections with the medial thalamic nuclei and receives a direct input from the ACC. Neural terminations arising from medial thalamic nuclei are ideal for providing motivational and affective qualities associated with noxious stimulation. Thus, these multiple projections and terminations provide a material basis for the multidimensional nature of pain and complex relationship to injury that is captured by the biopsychosocial model .
Functional imaging during noxious stimulation
Many studies have examined the brain response to acute pain in healthy subjects using positron emission tomography, functional MRI, magnetoencephalography, and electroencephalography and are reviewed extensively elsewhere . Similar techniques also have been extended to the study of chronic pain disorders, such as irritable bowel syndrome, fibromyalgia, atypical facial pain, and nonspecific low back pain. The persistence, intractability, and apparent absence of peripheral disease to account for these so-called “functional” pain disorders has led to particular interest in the possibility of a central etiology and the use of functional imaging to test central hypotheses. Figs. 1 and 2 summarize the responses observed in healthy controls and patients who have functional pain on the lateral (see Fig. 1 ) and medial (see Fig. 2 ) surfaces of the brain.