Traumatic stress has a broad range of effects on the brain. Brain areas implicated in the stress response include the amygdala, the hippocampus, and the prefrontal cortex. Studies in patients who have posttraumatic stress disorder (PTSD) and other psychiatric disorders related to stress have replicated findings in animal studies by finding alterations in these brain areas. Brain regions implicated in PTSD also play an important role in memory function, highlighting the important interplay between memory and the traumatic stress response. Abnormalities in these brain areas are hypothesized to underlie symptoms of PTSD and other stress-related psychiatric disorders.
Effects of traumatic stress on the individual
Traumatic stressors, including childhood abuse, can lead to posttraumatic stress disorder (PTSD), depression , substance abuse , dissociative disorders , personality disorders , and health problems . For many trauma victims, PTSD, which affects about 8% of Americans at some time in their lives , may be a life-long problem . However, the development of effective treatments is limited by gaps in knowledge about the underlying neurobiologic mechanisms that mediate symptoms of trauma-related disorders like PTSD. Until 12 years ago, no brain imaging studies had been performed in patients who had PTSD or other stress-related psychiatric disorders. The past decade has seen an explosion of research using brain imaging to assess the effects of traumatic stress on the brain . These studies have implicated the amygdala, the hippocampus, and the medial prefrontal cortex (including anterior cingulate) in PTSD and other stress-related psychiatric disorders. This article reviews brain imaging studies, looking at the effects of traumatic stress on the brain, and integrates them with basic scientific findings on the neuroscience of stress.
Neural circuits of posttraumatic stress disorder
PTSD is characterized by specific symptoms, including intrusive thoughts, hyperarousal, flashbacks, nightmares and sleep disturbances, changes in memory and concentration, and startle responses. Symptoms of PTSD are hypothesized to represent the behavioral manifestation of stress-induced changes in brain structure and function. Stress results in acute and chronic changes in neurochemical systems and specific brain regions, which result in long-term changes in brain “circuits” involved in the stress response . Brain regions that are felt to play an important role in PTSD include the hippocampus, the amygdala, and the medial prefrontal cortex.
Preclinical and clinical studies have shown alterations in memory function following traumatic stress , and changes in a circuit of brain areas, including the hippocampus, the amygdala, and the medial prefrontal cortex, that mediate alterations in memory . The hippocampus, which is involved in verbal declarative memory, is very sensitive to the effects of stress. Stress in animals has been associated with damage to neurons in the CA3 region of the hippocampus (which may be mediated by hypercortisolemia, decreased brain derived neurotrophic factor, or elevated glutamate levels) and inhibition of neurogenesis .
Antidepressant treatments have been shown to block the effects of stress and to promote neurogenesis . Animal studies have demonstrated several agents with potentially beneficial effects on stress-induced hippocampal damage. It has been found that phenytoin blocks the effects of stress on the hippocampus, probably through modulation of excitatory amino acid–induced neurotoxicity . Other agents, including tianeptine, dihydroepiandrosterone, and fluoxetine have similar effects . These medications may share a common mechanism of action through up-regulation of cyclic adenosine monophosphate (cAMP) response element–binding protein, which leads to regulation of expression of specific target genes involved in structural modeling of the hippocampus. Such treatment effects on brain-derived neurotrophic factor and its receptor trkB mRNA can have long-term effects on brain structure and function. New evidence suggests that neurogenesis is necessary for the behavioral effects of antidepressants , although this continues to be a source of debate .
In addition to the hippocampus, other brain structures have been implicated in a neural circuitry of stress, including the amygdala and prefrontal cortex. The amygdala is involved in memory for the emotional valence of events, and plays a critical role in the acquisition of fear responses . The medial prefrontal cortex includes the anterior cingulate gyrus (Brodmann’s area [BA] 32) and subcallosal gyrus (BA 25) and the orbitofrontal cortex. Lesion studies demonstrated that the medial prefrontal cortex modulates emotional responsiveness through inhibition of the amygdala function . Studies show that neurons of the medial prefrontal cortex play an active role in inhibition of fear responses that are mediated by the amygdala . Conditioned fear responses are extinguished following repeated exposure to the conditioned stimulus (CS) (in the absence of the unconditioned aversive [eg, electric shock] stimulus). This inhibition appears to be mediated by medial prefrontal cortical inhibition of amygdala responsiveness. Animal studies have also shown that early stress is associated with a decrease in branching of neurons in the medial prefrontal cortex . The insula also plays a critical role in integrating the physiologic stress response.
Neural circuits of posttraumatic stress disorder
PTSD is characterized by specific symptoms, including intrusive thoughts, hyperarousal, flashbacks, nightmares and sleep disturbances, changes in memory and concentration, and startle responses. Symptoms of PTSD are hypothesized to represent the behavioral manifestation of stress-induced changes in brain structure and function. Stress results in acute and chronic changes in neurochemical systems and specific brain regions, which result in long-term changes in brain “circuits” involved in the stress response . Brain regions that are felt to play an important role in PTSD include the hippocampus, the amygdala, and the medial prefrontal cortex.
Preclinical and clinical studies have shown alterations in memory function following traumatic stress , and changes in a circuit of brain areas, including the hippocampus, the amygdala, and the medial prefrontal cortex, that mediate alterations in memory . The hippocampus, which is involved in verbal declarative memory, is very sensitive to the effects of stress. Stress in animals has been associated with damage to neurons in the CA3 region of the hippocampus (which may be mediated by hypercortisolemia, decreased brain derived neurotrophic factor, or elevated glutamate levels) and inhibition of neurogenesis .
Antidepressant treatments have been shown to block the effects of stress and to promote neurogenesis . Animal studies have demonstrated several agents with potentially beneficial effects on stress-induced hippocampal damage. It has been found that phenytoin blocks the effects of stress on the hippocampus, probably through modulation of excitatory amino acid–induced neurotoxicity . Other agents, including tianeptine, dihydroepiandrosterone, and fluoxetine have similar effects . These medications may share a common mechanism of action through up-regulation of cyclic adenosine monophosphate (cAMP) response element–binding protein, which leads to regulation of expression of specific target genes involved in structural modeling of the hippocampus. Such treatment effects on brain-derived neurotrophic factor and its receptor trkB mRNA can have long-term effects on brain structure and function. New evidence suggests that neurogenesis is necessary for the behavioral effects of antidepressants , although this continues to be a source of debate .
In addition to the hippocampus, other brain structures have been implicated in a neural circuitry of stress, including the amygdala and prefrontal cortex. The amygdala is involved in memory for the emotional valence of events, and plays a critical role in the acquisition of fear responses . The medial prefrontal cortex includes the anterior cingulate gyrus (Brodmann’s area [BA] 32) and subcallosal gyrus (BA 25) and the orbitofrontal cortex. Lesion studies demonstrated that the medial prefrontal cortex modulates emotional responsiveness through inhibition of the amygdala function . Studies show that neurons of the medial prefrontal cortex play an active role in inhibition of fear responses that are mediated by the amygdala . Conditioned fear responses are extinguished following repeated exposure to the conditioned stimulus (CS) (in the absence of the unconditioned aversive [eg, electric shock] stimulus). This inhibition appears to be mediated by medial prefrontal cortical inhibition of amygdala responsiveness. Animal studies have also shown that early stress is associated with a decrease in branching of neurons in the medial prefrontal cortex . The insula also plays a critical role in integrating the physiologic stress response.
Changes in brain structure in posttraumatic stress disorder and stress-related disorders
Studies have demonstrated several consistent changes in cognition and brain structure associated with PTSD, including verbal declarative memory deficits . Patients who had PTSD secondary to combat and childhood abuse have been reported to have deficits in verbal declarative memory function based on neuropsychologic testing. Using various measures, including the Wechsler Memory Scale, the visual and verbal components of the Selective Reminding Test, the Auditory Verbal Learning Test, Paired Associate Recall, the California Verbal New Learning Test, and the Rivermead Behavioral Memory Test, investigators have found specific deficits in verbal declarative memory function with a relative sparing of visual memory and IQ . These studies have been conducted in patients who had PTSD related to various causes, including Vietnam combat (see Refs. ), rape , the Holocaust , early childhood abuse , and trauma as children . Returning Iraq soldiers were shown to have diminished verbal memory performance compared with their predeployment baselines, with greater verbal memory deficits in veterans with high levels of PTSD symptoms . These findings suggest that traumas such as early abuse with associated PTSD result in deficits in verbal declarative memory.
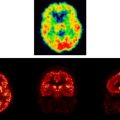
Several studies of PTSD have shown changes in hippocampal volume associated with the disorder. The author’s group first demonstrated this finding in Vietnam veterans with PTSD, who had an 8% reduction in right hippocampal volume based on MR imaging, relative to controls matched for various factors including alcohol abuse and education ( P <.05); smaller volume was correlated with deficits in verbal declarative memory function as measured by the Wechsler Memory Scale ( Fig. 1 ) . A second study from the author’s group showed a 12% reduction in mean left hippocampal volume in 17 patients who had childhood abuse-related PTSD, compared with 17 case-matched controls; this group difference was significant after controlling for confounding factors . Smaller hippocampal volume has been shown to be specific to PTSD within the anxiety disorders, and has not been demonstrated in panic disorder . Gurvits and colleagues showed bilateral hippocampal volume reductions in combat-related PTSD, compared with combat veterans without PTSD and normal controls. Combat severity was correlated with volume reduction. Stein and colleagues found a 5% reduction in left hippocampal volume. Other studies of PTSD also have found smaller hippocampal volume or reductions in N-acetylaspartate (NAA), a marker of neuronal integrity . The author’s group has reported smaller hippocampal volume in PTSD subjects compared with trauma-exposed non-PTSD subjects , whereas other investigators have observed reductions in both trauma-exposed non-PTSD and trauma-exposed PTSD subjects, relative to healthy comparison subjects . Studies in childhood PTSD did not find hippocampal volume reduction, although reduced NAA was found in the medial prefrontal cortex in childhood PTSD . Although some studies of new-onset or recent PTSD have not found changes in hippocampal volume , others have showed reductions . In a recent meta-analysis, the author’s group pooled data from all of the published studies and found smaller hippocampal volume for both the left and the right sides, equally in adult men and women with chronic PTSD, and no change in children . Another recent meta-analysis had similar findings . More recent studies of Holocaust survivors with PTSD did not find a reduction in hippocampal volume , although subjects who developed PTSD in response to an initial trauma had smaller hippocampal volume, compared with those who developed PTSD after repeated trauma, suggesting that a small hippocampal volume may impart vulnerability . Several studies have shown that PTSD patients have deficits in hippocampal activation while performing a verbal declarative memory task or a virtual water maze task . Hippocampal atrophy and hippocampal-based memory deficits reversed with treatment with the selective serotonin reuptake inhibitor (SSRI) paroxetine, which has been shown to promote hippocampal neurogenesis in preclinical studies . The author hypothesizes that stress-induced hippocampal dysfunction may mediate many of the symptoms of PTSD that are related to memory dysregulation, including explicit memory deficits and fragmentation of memory in abuse survivors. It is unclear at the current time whether these changes are specific to PTSD, whether certain common environmental events (eg, stress) in different disorders lead to similar brain changes, or whether common genetic traits lead to similar outcomes.
Several studies have found smaller anterior cingulate volumes based on MR imaging measurements in PTSD , including women who experienced abuse and PTSD . One study found a reduction in the ratio of NAA to creatinine measured with MR spectroscopy , whereas another found a decrease in gray matter density . An important question is whether these effects are reversible with treatment. Other findings related to volumetrics include smaller volumes of the corpus callosum in neglected children and adults with PTSD . One study showed smaller volumes of the insula with voxel-based morphometry . A study in twins found smaller volumes of the cavum septum pellucidum .
Functional neuroimaging studies in posttraumatic stress disorder
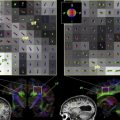
Imaging studies of brain function in PTSD implicate dysfunction of the medial prefrontal cortex, amygdala, and hippocampus . Methodologies used in imaging studies of PTSD are outlined in Table 1 and a summary of findings by investigator and brain region appears in Table 2 . Studies of resting cerebral blood flow or metabolism with positron emission tomography (PET) and single photon emission tomography (SPECT) have shown alterations at rest in the medial prefrontal, temporal, and dorsolateral prefrontal cortices, cerebellum, and amygdala . Stimulation of the noradrenergic system with yohimbine resulted in a failure of activation in the dorsolateral prefrontal, temporal, parietal, and orbitofrontal cortex, and decreased function in the hippocampus . Exposure to traumatic reminders in the form of traumatic slides or sounds or traumatic scripts have been associated with an increase in PTSD symptoms, decreased cerebral blood flow, or a failure of activation in the medial prefrontal cortex/anterior cingulate, including BA 25, or subcallosal gyrus, BA 32, and BA 24, as measured with PET, SPECT or functional MR (fMR) imaging ( Fig. 2 ) . Other findings in studies of traumatic reminder exposure include decreased function in the hippocampus , thalamus , visual association cortex , parietal cortex , and inferior frontal gyrus (see Refs. ), and increased function in the amygdala , posterior cingulate , and parahippocampal gyrus . Shin and colleagues found a correlation between increased amygdala function and decreased medial prefrontal function with traumatic reminders, indicating that a failure of inhibition of the amygdala by the medial prefrontal cortex could account for increased PTSD symptoms with traumatic reminders. Other studies have found increased amygdala and parahippocampal function and decreased medial prefrontal function during the performance of an attention task , and increased amygdala function at rest , during a working memory task , during recall of traumatic words , and with exposure to masked fearful faces , overt fearful faces , traumatic sounds , and traumatic scripts .
| Authors | Study population | SampleSize | Control group | Samplesize | Imaging methods | Active condition | Control |
|---|---|---|---|---|---|---|---|
| Rauch | Mixed PTSD | 8 | None | 0 | PET O-15 | Combat scripts | Neutral scripts |
| Semple | Combat-related PTSD + SA | 6 | Healthy subjects | 6 | PET FDG | Continuous performance task | Rest |
| Bremner | Combat-related PTSD | 10 | Healthy subjects | 10 | PET FDG | Yohimbine | Placebo |
| Shin | Combat-related PTSD | 7 | Combat veterans without PTSD | 7 | PET O-15 | Trauma imagery/perception | Negative/neutral imagery/perception |
| Bremner | Combat-related PTSD | 10 | Combat veterans without PTSD | 10 | PET O-15 | Combat slides/sounds | Neutral slides/sounds |
| Bremner | Women with abuse-related PTSD | 10 | Abused women without PTSD | 12 | PET O-15 | Abuse scripts | Neutral scripts |
| Shin | Women with abuse-related PTSD | 8 | Abused women without PTSD | 8 | PET O-15 | Abuse scripts | Neutral scripts |
| Liberzon | Combat-related PTSD | 14 | Healthy subjects/combat controls | 14/11 | SPECT HMPAO | Combat sounds | White noise |
| Zubieta | Combat-related PTSD | 12 | Combat veterans without PTSD, healthy subjects | 11/12 | SPECT HMPAO | Combat sounds | White noise |
| Rauch | Combat-related PTSD | 8 | Combat veterans without PTSD | 8 | fMR imaging | Masked fearful faces | Masked happy faces |
| Semple | Combat-related PTSD + SA | 6 | Healthy subjects | 7 | PET O-15 butanol | Continuous performance task | Rest |
| Shin | Combat-related PTSD | 8 | Combat veterans without PTSD | 8 | fMR imaging | Counting Stroop-combat | Stroop general negative |
| Lanius | Mixed civilian (SA or MVA) | 9 | Traumatized non-PTSD subjects | 9 | fMR imaging | Traumatic scripts | Resting state |
| Pissiota | Combat-related PTSD | 7 | None | 0 | PET | Traumatic sounds | Neutral sounds |
| Lanius | Mixed civilian (SA or MVA) | 10 | Traumatized non-PTSD subjects | 10 | fMR imaging | Sad, anxious, trauma scripts | Resting state |
| Bremner | Women with abuse-related PTSD | 10 | Healthy women | 11 | PET O-15 | Trauma-related word recall | Shallow encoding |
| Bremner | Women with abuse-related PTSD | 10 | Women with abuse without PTSD | 12 | PET O-15 | Memory task | Shallow encoding |
| Clark | Civilian PTSD | 10 | Healthy subjects | 10 | PET O-15 | Working memory task | Fixed target |
| Bonne | Civilian PTSD | 11 | Trauma controls/healthy controls | 17/11 | SPECT HMPAO | Resting state | — |
| Bremner | Women with abuse-related PTSD | 8 | Healthy subjects | 11 | PET O-15 | Fear conditioning | Unpaired CS-UCS |
| Bremner | Women with abuse-related PTSD | 12 | Women with abuse without PTSD | 9 | PET O-15 | Emotional Stroop | Neutral Stroop |
| Shin | Vietnam combat-related PTSD | 17 | Vietnam veterans without PTSD | 19 | PET O-15 | Traumatic scripts | Neutral scripts |
| Shin | Firefighters with PTSD | 8 | Firefighters without PTSD | 8 | PET O-15 | Memory task | Shallow encoding |
| Lindauer | Policemen with PTSD | 15 | Policemen without PTSD | 15 | SPECT HMPAO | Traumatic scripts | Neutral scripts |
| Yang | Children with earthquake-related PTSD | 5 | Children with earthquake-related non-PTSD | 6 | fMR imaging | Earthquake pictures/imagery | Neutral pictures/imagery |
| Shin | Firefighters + Vietnam combat with PTSD | 13 | Trauma exposed without PTSD | 13 | fMR imaging | Overt fearful faces | Neutral overt faces |
| Armony | Acute PTSD: MVA | 13 | None | 0 | fMR imaging | Masked fearful faces | Masked happy faces |
| Sakamoto | Mixed civilian PTSD | 16 | Healthy subjects | 16 | fMR imaging | Masked traumatic images | Masked neutral images |
| Protopopescu | Sexual/physical abuse PTSD | 11 | Healthy subjects | 21 | fMR imaging | Traumatic word recall | Neutral word recall |
| Bryant | Civilian PTSD | 14 | Healthy controls | 14 | fMR imaging | Oddball working memory | — |
| Britton | Combat-related PTSD | 16 | Combat controls/healthy controls | 15/14 | PET O-15 | Traumatic scripts | Neutral scripts |
| Chung | Civilian PTSD | 23 | Healthy controls | 46 | SPECT HMPAO | Resting state | None |
| Phan | Vietnam combat-related PTSD | 16 | Combat controls/healthy subjects | 15/15 | PET | Negative pictures | Control pictures |
| Astur | Civilian PTSD | 12 | Healthy controls | 12 | fMR imaging | Virtual water maze | Visual condition |
Several studies have examined neural correlates of cognitive tasks in PTSD. During working memory tasks, patients showed decreased inferior frontal and parietal function . Retrieval of emotionally valenced words (eg, “rape-mutilate”) in women with PTSD from early abuse resulted in decreases in blood flow in an extensive area that included the orbitofrontal cortex, anterior cingulate, and medial prefrontal cortex (BA 9, 25, and 32), left hippocampus, and fusiform gyrus/inferior temporal gyrus, with increased activation in the posterior cingulate, left inferior parietal cortex, left middle frontal gyrus, and visual association and motor cortex . Another study found a failure of medial prefrontal cortical/anterior cingulate activation and decreased visual association and parietal cortex function during performance of the emotional Stroop task (ie, naming the color of a word such as “rape”) in women with PTSD who were abused, relative to abused women without PTSD . Shin and colleagues showed increased posterior cingulate and parahippocampal gyrus and decreased medial prefrontal and dorsolateral prefrontal during an emotional “counting” Stroop paradigm with fMR imaging.
Declarative memory tasks have been used as specific probes of the hippocampal function in PTSD. The author’s group measured brain activation with a paragraph-encoding task in conjunction with PET O-15 measurements of cerebral blood flow. Women with PTSD and a history of abuse showed a failure of hippocampal activation during the memory task, relative to control subjects . Women with PTSD who had been abused also had smaller hippocampal volumes as measured with MR imaging, relative to both abused women without PTSD and nonabused, non-PTSD women. The failure of hippocampal activation was significant after controlling for differences in hippocampal volume and accuracy of encoding. Shin and colleagues also found a failure of hippocampal activation with a memory stem completion task in PTSD.
Although multiple studies have used symptom provocation with traumatic scripts or similar designs, little has been done in the area of fear conditioning in PTSD. To that end, the author’s group studied women with a history of severe childhood sexual abuse and the diagnosis of current PTSD (N = 8) and women without childhood abuse or PTSD (N = 11). All subjects underwent PET measurements of cerebral blood flow and psychophysiologic measurements of heart rate and skin conductance during habituation, acquisition, and extinction conditions, on a single day, with scanning during a control condition on another day separated by 1 week from the active condition. During habituation, subjects were repeatedly exposed to a blue square on a screen. During active fear acquisition, exposure to the blue square (CS) was paired with an electric shock to the forearm (unconditioned stimulus [UCS]), and during extinction, subjects were again exposed to the blue squares (CS) without shock (“active” extinction). On a second day, subjects went through the same procedure, with electric shocks delivered randomly when the blue square was not present (unpaired CS-UCS). Acquisition of fear was associated with increased skin conductance responses to CS exposure during the active versus the control conditions in all subjects. The skin conductance response for PTSD was increased during the first CS-UCS presentation. Extinction of fear was associated with increased skin conductance responses to CS exposure during the active versus the control conditions in all subjects. When PTSD and non-PTSD subjects were examined separately, skin conductance response levels were significantly elevated in non-PTSD subjects undergoing extinction following the active, compared with the control, condition during session one. PTSD subjects showed activation of the bilateral amygdala during fear acquisition, compared with the control condition ( Fig. 3 ). Non-PTSD subjects showed an area of activation in the region of the left amygdala. When PTSD subjects and control subjects were compared directly, PTSD subjects showed greater activation of the left amygdala than healthy women during the fear conditioning condition (pairing of UCS and CS), relative to the random shock control. Other areas that showed increased activation with fear acquisition in PTSD included bilateral superior temporal gyrus (BA 22), cerebellum, bilateral inferior frontal gyrus (BA 44, 45), and posterior cingulate (BA 24). Fear acquisition was associated with decreased function in the medial prefrontal cortex, visual association cortex, and medial temporal cortex, the inferior parietal lobule function, and other areas. Extinction of fear responses was associated with decreased function in the orbitofrontal and medial prefrontal cortex (including subcallosal gyrus, BA 25, and anterior cingulate, BA 32), visual association cortex, and other areas in the PTSD subjects, but not in the controls. Amygdala blood flow with fear acquisition was negatively correlated with medial prefrontal blood flow with fear extinction (increased blood flow in amygdala correlated with decreased blood flow in medial prefrontal cortex) in all subjects (r = −0.48; P <.05). Increased amygdala blood flow with fear acquisition was positively correlated with PTSD (r = 0.45), anxiety (r = 0.44), and dissociative (r = 0.80) symptom levels in PTSD (but not non-PTSD) subjects. The medial prefrontal blood flow during extinction and anxiety were negatively correlated, as measured with the Panic Attack Symptom Scale during extinction in the PTSD group only, which was significant after correction for multiple comparisons (r = −0.90; P = .006) . This study was consistent with increased amygdala function with fear acquisition, and decreased medial prefrontal (anterior cingulate) function during extinction in PTSD. This finding is consistent with the model of an overactive amygdala and a failure of the medial prefrontal cortex to extinguish the amygdala when the acute threat is no longer present.