Parameter
Balanced gradient echo sequence (FIESTA, true FISP, SBSSFP)
T2 half-Fourier sequence (HASTE)
T1 3D FS gradient echo sequence
DWI
Axial
Coronal/sagittal
Axial/axial BFS
Coronal/sagittal
Axial/sagittal
Axial/sagittal
Repetition time/echo time (ms)
4.3/2.2
4.3/2.2
1000/90
1000/90
4.1/1.1
3200/75
Flip angle (0)
50
50
150
150
10
10
Field of view (mm)
320–400
320–400
320–400
320–400
320–400
320–400
Matrix
256 × 224
256 × 224
256 × 400
256 × 224
256 × 224
256 × 192
Parallel imaging factor
2
2
2
2
3
2
Section thickness (mm)
5
5
4
4
2.5
10
Intersection gap (mm)
0
0
0
0
0
0
NEX
1
1
1
1
1
6
Receiver bandwidth
125
125
62.50
62.50
62.50
1930
Table 20.2
Suggested MR imaging protocols in pregnancy as determined by clinical question
Clinical question | Suggested sequences |
|---|---|
Cervical cancer in pregnancy – assessment and staging | Axial T1 abdomena Axial and sagittal T2 pelvisb Axial oblique T2 pelvis Coronal T1FSc Axial and sagittal DWI 50, 400, 800 |
Leiomyoma in pregnancy – assessment | Axial T1 abdomen Axial and sagittal T2 pelvis Axial oblique T2 pelvis Axial and coronal T1FS Axial DWI 50, 400, 800 |
Adnexal mass characterisation | Axial T1 abdomen Axial T2 pelvis Axial oblique T2 pelvis Axial and coronal T1FS Axial DWI 50, 400, 800 |
20.3 Normal Appearances of the Uterus, Cervix and Adnexa During Pregnancy
Cervix
The MR appearances of the normal cervix change as pregnancy progresses. As gestational age increases, the cross-sectional area of the cervix increases (31 % increase in the cross-sectional area of the cervical stroma by 12/40) [23]. The length of the cervix will exceed 3 cm. The T2W SI of the external os is greater than of the internal os, and the signal intensity of the outer stroma is greater than the inner stroma [8, 23, 40] (Fig. 20.1).
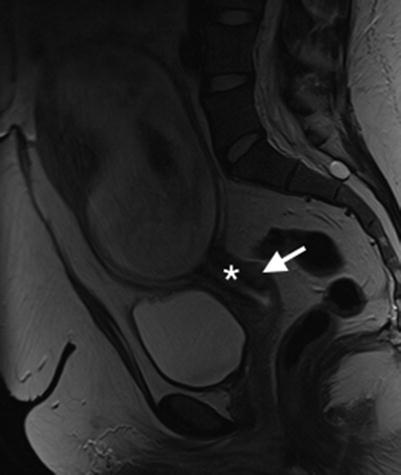

Fig. 20.1
Normal cervix and gravid uterus. Sagittal T2W image of the normal cervix in pregnancy. Note that signal intensity of the outer stroma (arrow) is greater than the inner stroma (asterisk)
Ovaries
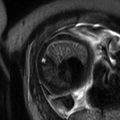
The normal ovary in pregnancy has a low T2 signal intensity (T2SI) stroma and contains small follicles (Fig. 20.2). As pregnancy progresses the ovaries are displaced anteriorly and superiorly by the gravid uterus.
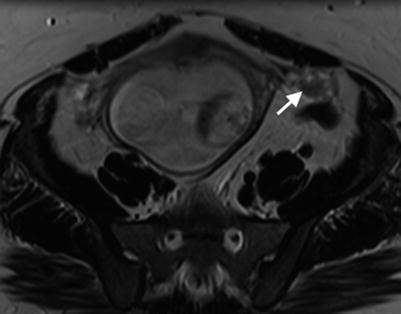

Fig. 20.2
Normal ovaries and gravid uterus. Axial T2W image of the pelvis showing a normal left ovary (arrow) displaced anteriorly and superiorly by the gravid uterus (18/40)
Myometrium
The myometrium returns intermediate signal intensity on T2W sequences, and the uterine wall is readily visible. Thin slices performed perpendicular to the myometrial-placental junction reduce partial voluming and allow distinction between the myometrium and placenta (a common pitfall with thicker slices).
20.4 Cervical Cancer in Pregnancy
Cervical cancer is the most commonly diagnosed gynaecologic malignancy during pregnancy. Incidence rates vary from 0.1 to 12 per 10,000 pregnancies [1].
Presentation of cervical cancer during pregnancy is the same as for non-pregnant patients, usually with painless post-coital bleeding. However, symptoms can be mistaken for complications of pregnancy, and this can lead to delay in diagnosis if clinical suspicion is low [34]. Cervical cancer does not adversely affect pregnancy, and pregnancy does not alter the course of the cancer.
MR is the single best imaging investigation that can accurately determine cervical tumour location, tumour size, depth of stromal invasion and extension into the lower uterine segment to assess local disease extent [48]. Accurate pretreatment evaluation of these (prognostic) factors is crucial in determining appropriate therapy.
Cervical cancer staging is based on the FIGO clinical and pathological staging system (Table 20.3) [41]. On T1W images, tumours are isointense to the normal cervix and are difficult to visualise. On T2W imaging, cervical cancer appears as a relatively hyperintense mass, easily distinguishable from low signal intensity cervical stroma (Fig. 20.3). Large tumours may be necrotic. MR features of cervical cancer in pregnant patients should be comparable to the non-pregnant patient [35].
FIGO stage | TNM stage | Extent of disease |
|---|---|---|
– | Tis | In situ |
I | T1 | Confined to uterus |
IA | T1a | Diagnosed only by microscopy |
IA1 | T1a1 | Depth ≤3 mm, horizontal spread ≤7 mm |
IA2 | T1a2 | Depth >3–5 mm, horizontal spread ≤7 mm |
IB | T1b | Clinically visible or microscopic lesion greater than T1a2 |
IB1 | T1b1 | ≤4 cm |
IB2 | T1b2 | >4 cm |
II | T2 | Beyond uterus but not pelvic wall or lower third of vagina |
IIA | T2a | No parametrium |
IIA1 | T2a1 | ≤4 cm |
IIA2 | T2a2 | >4 cm |
IIB | T2b | Parametrium |
III | T3 | Lower third vagina/pelvic wall/hydronephrosis |
IIIA | T3a | Lower third of vagina |
IIIB | T3b | Pelvic wall/hydronephrosis |
IVA | T4 | Mucosa of bladder/rectum; beyond true pelvis |
N1 | Regional | |
IVB | M1 | Distant metastasis |

Fig. 20.3
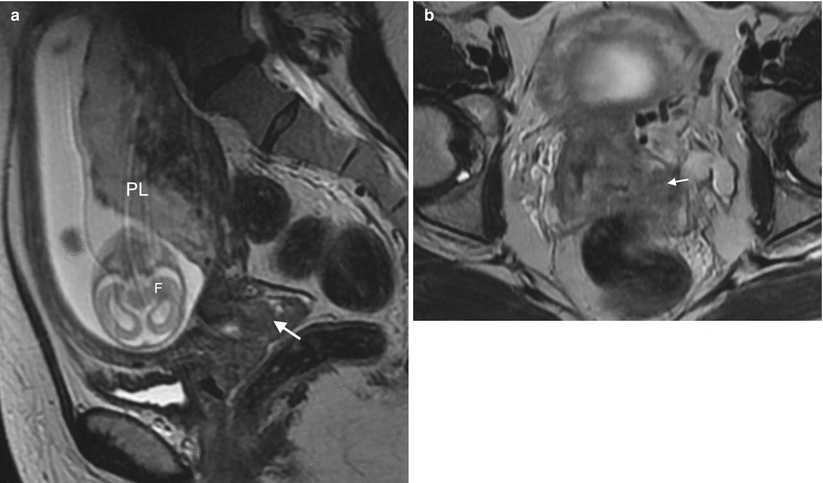
Cervix cancer in gravid uterus. (a) Sagittal T2W image of intermediate signal intensity barrel-shaped tumour of the cervix (arrow). (b) Axial oblique T2W image of the cervical tumour demonstrating left parametrial invasion (arrow). PL placenta, F foetus
However, it is important to note that the MR appearances of the normal cervix change as pregnancy progresses, and this can complicate interpretation when staging a cervical cancer. As described above the cross-sectional area of the cervix increases throughout pregnancy, the signal intensity of the external os is greater than the internal os and the signal intensity of the outer stroma is greater than the inner stroma [8, 23, 40]. Furthermore the MR signal intensity increases with gestational age and is maximal pre-partum. Therefore, in pregnancy the tumour may appear either isointense or hypointense against the physiological hyperintensity of the cervix. Nodal staging in the pelvis and para-aortic regions can be undertaken on MRI with moderate accuracy only. The presence of prominent pelvic veins due to the pregnancy may cause some difficulty in the interpretation of the pelvic lymph nodes and parametrial invasion.
Imaging for distant metastatic disease is planned depending on the stage of pregnancy and extent of cervical disease. In the future, whole body MRI may be used to investigate distant metastases (if the patient is able to tolerate the scan).
Traditionally, the treatment of cervical cancer in pregnancy was avoided, and the management strategy was termination of pregnancy if detected during the first two trimesters or delay of treatment until foetal maturity in the third trimester. Standard treatment was initiated post-partum. More recently, however, pregnancy preservation and treatment during pregnancy have become more common. Decision to treat and treatment schema clearly depend on disease stage, and the role of imaging in this setting is paramount to facilitate accurate staging and assessment of treatment response [21, 34].
Given the rarity of the disease and the complicated factors that must be taken into consideration on a case-by-case basis, standardisation of treatment is a challenge, and current guidelines are based on small case series and expert opinion. When a patient with cervical cancer presents, several issues must be considered by the multidisciplinary team – disease stage, nodal status, gestational age, obstetric complications and importantly the patient’s wishes regarding continuation versus termination of pregnancy [21].
20.4.1 Considerations Post-Fertility-preserving Surgery for Cervical Cancer
Historically patients with invasive cervical cancer stage IA up to IIA would be treated with a traditional Wertheim radical hysterectomy or radiation therapy. Higher-stage disease with parametrial invasion is treated with chemoradiation therapy. Although these radical therapies have good survival, they are associated with subsequent sterility. In recent years, surgical techniques have explored radical tumour resection without hysterectomy in young women with early-stage disease, thereby preserving fertility as well as the uterus and ovarian function. Radical trachelectomy with pelvic lymphadenectomy is a conservative but curative surgical procedure for early (stage 1B1 or lower) carcinoma of the cervix [47]. The surgical technique varies, but typically involves resection of the cervix (together with variable parametrial resection) and upper vaginal vault, and then anastomosis of the residual corpus uteri to the remainder of the vaginal vault (Fig. 20.4a). A cerclage suture is then placed around the corpus uteri at the anastomosis to maintain competency of the uterus in any subsequent pregnancies. Cerclage sutures may cause susceptibility artefact on MRI (Fig. 20.4b).
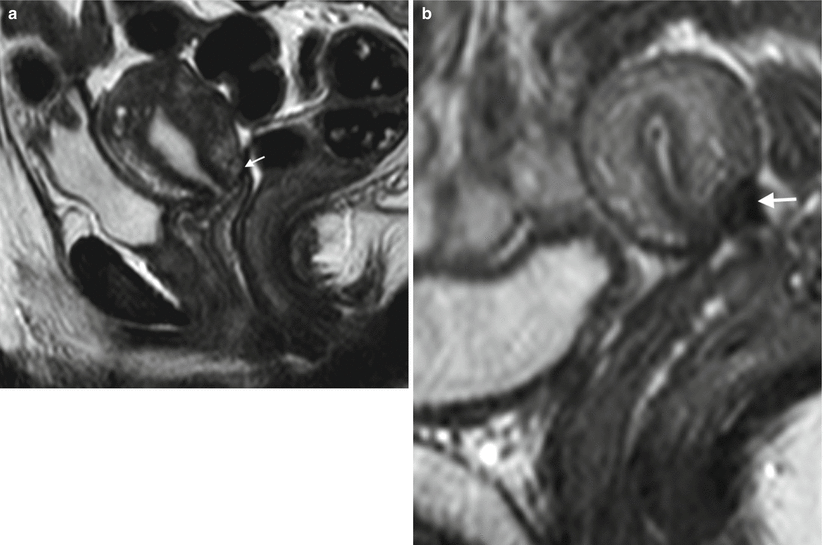

Fig. 20.4
Post-trachelectomy appearances. (a) Sagittal T2W image of a patient who underwent trachelectomy for stage 1B grade 1 adenocarcinoma of the cervix. Note the end-to-end anastomosis of the uterine body and vaginal vault (arrow). (b) Sagittal T2W image in a different patient who underwent trachelectomy demonstrates prominent susceptibility artefact from cerclage suture (arrow)
Several studies celebrate successful pregnancy outcomes for patients who have undergone trachelectomy [6, 45, 46, 47] but caution that there is an increased incidence of preterm premature rupture of the membranes, and therefore these pregnancies should be managed as high risk. Delivery should be by caesarian section.
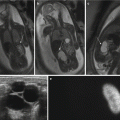
Other complications may include an increased risk of miscarriage and bleeding. At our institution we managed a patient who presented at 12/40 with PV bleeding who had undergone trachelectomy for cervical cancer 6 years previously (Fig. 20.4). The bleeding resolved spontaneously, and there was no MR evidence of recurrence on follow-up imaging (Fig. 20.5). In this case, clinical examination in conjunction with MR was paramount.
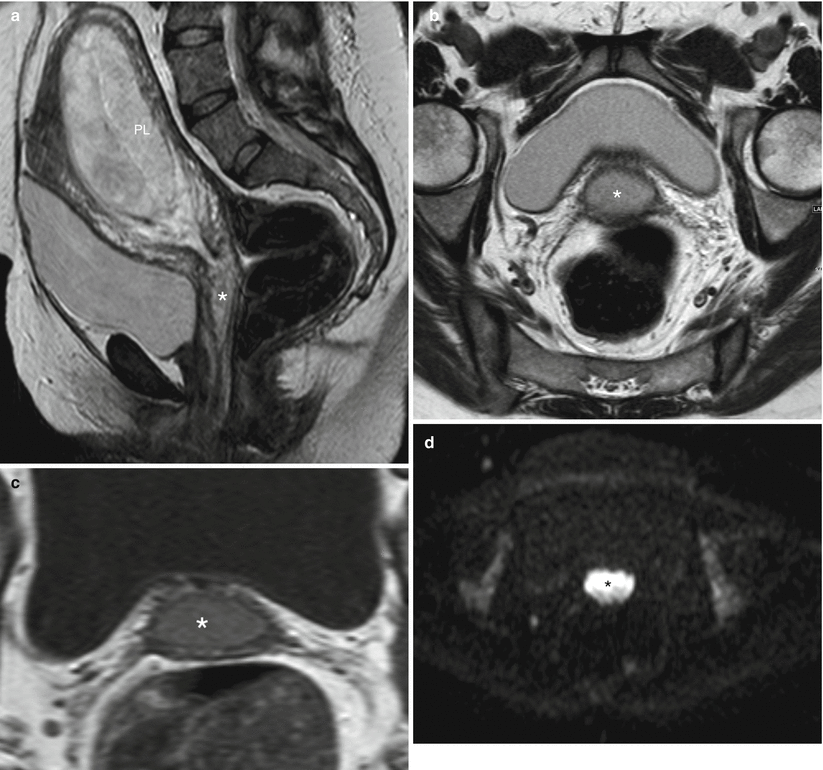
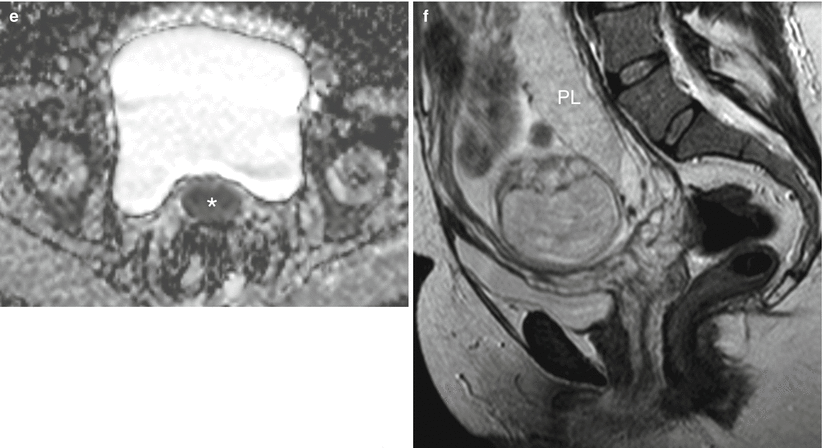


Fig. 20.5
Pregnancy post-trachelectomy with bleeding. (a) Following trachelectomy 6 years previously (Fig. 20.4a), this patient presented at 12/40 with vaginal bleeding. Sagittal T2W image demonstrates intermediate T2W signal material in the region of the cervix (asterisk) that is distending the vagina but not distorting its shape. (b) Axial oblique T2W image demonstrating intermediate T2W signal intensity ‘mass’ in the upper vagina (asterisk). (c) Axial T1W image where the ‘mass’ returns intermediate T1W signal (asterisk). (d, e) B 1200 image and corresponding ADC map demonstrate restricted diffusion within the ‘mass’ (asterisk). On clinical examination there was no visible recurrence, and these imaging appearances represent a blood clot. Repeat scan performed 6 weeks later (f) (T2W sagittal image) was normal, and the ‘mass’ was no longer visible. In this case clinical evaluation and imaging were vital and complementary to avoid pitfalls, exclude recurrence and identify complications. PL placenta
20.5 Adnexal Masses in Pregnancy
Adnexal masses are found in 1–2 % of pregnancies [44]. US is the first-line imaging modality and usually provides sufficient information for the treatment decision to be taken [7, 11]. Management is conservative or surgical depending on the imaging appearances of the mass, nature of presentation, gestational age, size of mass and patient preference. MR imaging may be helpful for characterisation of adnexal masses that remain indeterminate following US evaluation, for determining the origin of a pelvic mass and for surgical planning. In the acute setting, MR is particularly useful for evaluating the haemorrhagic content of adnexal masses, but clearly it should not delay surgical intervention. MR characteristics of adnexal masses that suggest benignity include high signal intensity on T1W sequences (indicating either fat, blood, or proteinaceous/mucinous content) with subsequent loss of signal intensity on fat-suppressed sequences (indicating fat), high signal intensity on T1W fat-suppressed images (indicative of blood) and low signal intensity on T2W images (indicative of fibrous tissue or haemosiderin) [32]. In particular, solid adnexal tissue that demonstrates low signal intensity on T2W sequences with low signal intensity on high b-value diffusion sequences has been shown to be highly suggestive of a benign non-invasive lesion [53]. In clinical practice, repeat imaging after pregnancy can be performed with gadolinium contrast enhancement to further support the diagnosis.
20.5.1 Cystic Adnexal Lesions
20.5.1.1 Simple and Haemorrhagic Cysts
Incidental US detection of a simple cyst in pregnancy is common. Size is the best indicator of whether the cyst requires monitoring or intervention as simple cysts that are smaller than 5 cm will resolve spontaneously in 90–100 % of cases [54]. Corpus luteal cysts enlarge during the first trimester, regress by the 12th week of gestation and disappear later on in the pregnancy. Haemorrhagic corpus luteal cysts have variable but characteristic US appearances due to the changing nature of the internal blood clot.
The decision to proceed to MR imaging in the context of a simple adnexal cyst is taken on a case-by-case basis. Larger cysts have an increased risk of torsion, rupture and obstetric complications, and in such cases MR is useful for accurate localisation and assessment of the position of the cyst and its relation to other structures. Simple ovarian cysts have thin featureless walls and return low signal intensity on T1W sequences and high signal intensity on T2W images. Corpus luteal cysts have thicker walls than simple cysts and have increased vascularity within the thickened wall seen as increased Doppler flow on US. They display more varied signal intensity on MR images because they are frequently haemorrhagic. MR is seldom required to aid diagnosis, but when used, haemorrhagic cysts return high signal intensity on T1W sequences and low signal intensity on heavily T2W sequences owing to T1 and T2 shortening. Haemorrhagic content is also demonstrated as hyperintense signal on T1 fat-saturated sequences [15, 37]. Adherent clot within a haemorrhagic corpus luteal cyst may mimic a solid nodule or papillary projection and raise concern regarding malignancy. In these cases the absence of a contrast-enhanced sequence makes this distinction challenging on MRI. Diffusion-weighted sequences can be helpful in this situation as, if the solid components return low SI on high b-value images, the likelihood of benignity is high. If adherent clot is suspected but not fully resolved on MRI and US, patients should be followed up with US to look for evolution and resolution of the haemorrhagic lesion.
20.5.1.2 Hyperstimulated Ovaries
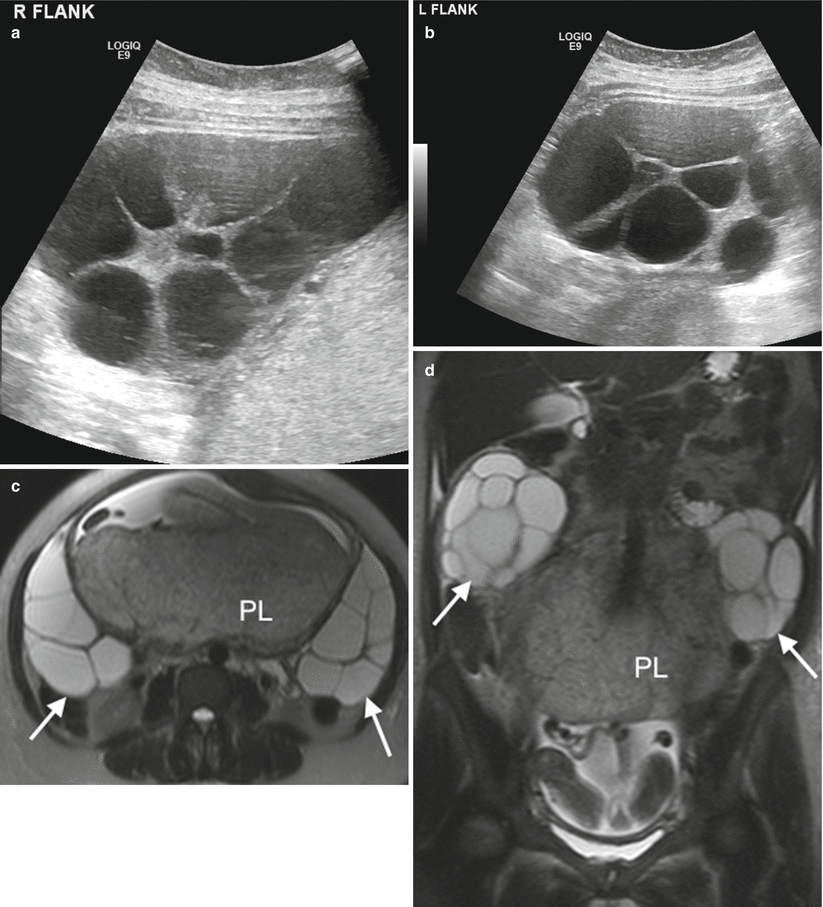
Typically seen in patients who have undergone ovarian induction, the ovaries are enlarged with multiple cysts. These will resolve spontaneously in more than 90 % of patients [11]. Ovarian hyperstimulation syndrome (OHSS) appears as markedly enlarged ovaries with extravascular accumulation of fluid resulting in ascites, pleural effusions, intravascular volume depletion and dehydration. The enlarged ovaries are at risk of torsion and haemorrhage, but they usually regress later in pregnancy or after delivery. MR may be considered in the context of ovarian hyperstimulation syndrome to exclude ovarian torsion and differentiate from ovarian tumour. Hyperstimulated ovaries demonstrate a ‘spoke-wheel’ appearance with enlarged follicles peripherally located and central ovarian stroma [24]. The follicles return bilateral symmetrical low signal intensity on T1W sequences (high T1 signal intensity in some foci may be in keeping with haemorrhage). On T2W sequences, multiple cysts will return homogenous high signal intensity. Central ovarian stroma will return intermediate to low T2 signal (higher if oedematous). Ascites will be high signal on T2. Treatment is usually conservative.
20.5.1.3 Hyperreactio Luteinalis
Appearances of hyperreactio luteinalis are similar to that of hyperstimulated ovaries, but this rare condition is seen in patients who have not undergone ovulation induction. It is thought to result from hypersensitivity of the ovary to circulating human chorionic gonadotropin (hCG). Symptoms may be absent or can include maternal abdominal pain, abdominal distension, abnormal liver function tests, respiratory difficulties and hirsutism. MR can be used to distinguish hyperstimulated ovaries from ovarian neoplasm. Appearances of the ovaries are similar to OHSS but without ancillary extravasation of fluid, therefore no significant ascites or pleural effusion (Fig. 20.6).