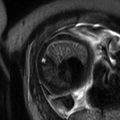
Fig. 15.1
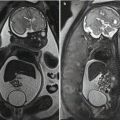
PRES in woman of 28 years old on the 7th month of pregnancy. The patient complains of seizures and hypertension. In FLAIR images (a) bilateral and symmetric hyperintense areas in parietal-occipital regions were evident. In (b) the follow-up exam (after 10 days) is evident with normalization of cerebral tissue intensity
Involvement is usually symmetric, but the degree of involvement may be asymmetric [2] (Fig. 15.2a–h).
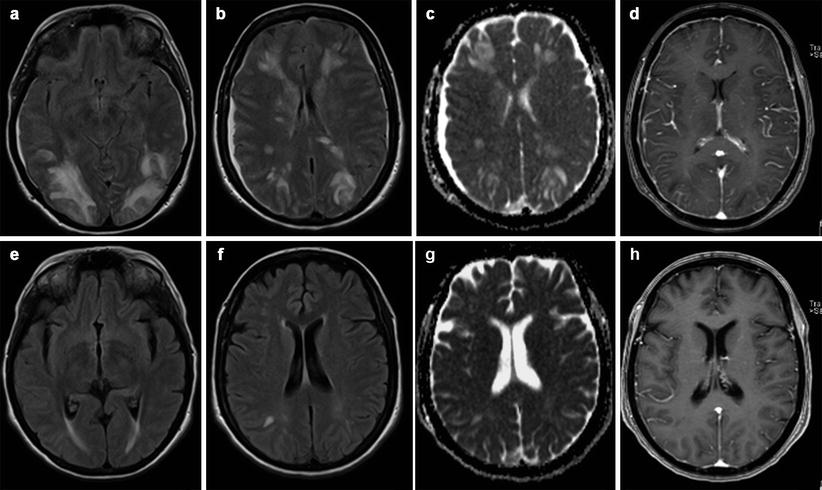

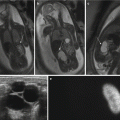
Fig. 15.2
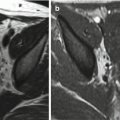
PRES and low CSF pressure syndrome after delivery and spinal anesthesia. Woman of 30 years old. Three days after delivery, the patient complains of seizures and orthostatic headaches. In MR images, there is evident cerebral vasogenic edema in the parietal-occipital and, to a lesser extent, in the frontal lobes bilaterally (a–c). Reduced ventricular volume and dural enhancement after gadolinium (d) are evident. After 15 days, a disappearance of brain lesions and a normalization of ventricular volume are noted (e–h)
Signal alterations can also occur to a lesser extent in the basal ganglia, posterior temporal and frontal lobes, corona radiate, brain stem, and cerebellum [6, 7] (Fig. 15.3a–e).
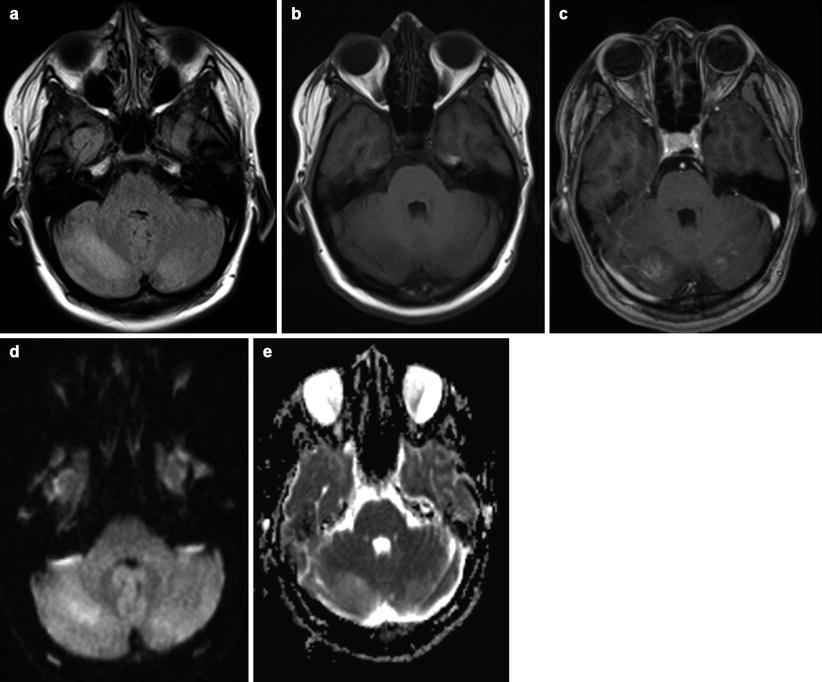

Fig. 15.3
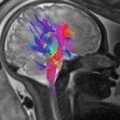
Cerebellar location of PRES in woman of 30 years old on the 6th month of pregnancy. Vasogenic edema (a, b) in both cerebellar hemispheres is associated with light enhancement after gadolinium (c). No evidence of reduced diffusivity is visible (d, e)
Evaluation with diffusion-weighted images of patients with PRES show increased diffusion with elevated values of ADC due to the presence of vasogenic edema. Nevertheless, there have been described cases of coexistence of vasogenic with cytotoxic edema [6].
Even though most reports have underlined a white matter predilection, Casey et al. in a recent study showed that cortical lesions are more common than what is previously thought [7, 9].
Patients with seizures rarely had involvement of cortical matter; patients with autoimmune disease were more likely to have cerebellar involvement; patients with sepsis or active infections were more likely to have cortical involvement [10].
Comparing pregnant and nonpregnant patients, despite some clinical significant differences in pregnant women, headache and visual disturbance are more common than in nonpregnant patients [11]; no other significant differences are reported about the topographic distribution of cerebral lesion. In both group, common areas involved by the lesions are occipital and parietal lobes, followed by frontal and temporal regions [10].
Pathophysiology of PRES is still incompletely understood.
One proposed mechanism is that hypertension induced potentially reversible vasospasm, especially of posterior border zone territories, characterized by cytotoxic edema which can possibly evolve to ischemic lesions if not successfully treated [6]. Occasional angiographic evaluation of women with preeclampsia/eclampsia documented widespread intracranial vessel vasospasm [12]. On the other hand, several considerations are in contrast with this hypothesis. If we accept arteriolar vasospasm and distal ischemia as the principal alteration, the acute lowering of mean arterial pressure should further reduce perfusion to these regions; however, most patients respond well to antihypertensive therapy and ischemic infarctions are fairly rare [6].
An alternative theory, which has been best characterized in preeclampsia, eclampsia, and sepsis, implicates endothelial dysfunction [10].
The third and now widely accepted hypothesis considers the altered status of cerebral perfusion autoregulation.
Brain perfusion is maintained constant by an autoregulatory system consisting of a myogenic and a neurogenic response (sympathetic innervation). The transient disruption of the cerebral autoregulation with forced dilatation of arterioles causes leakage of serum through capillary walls into the cerebral interstitium [2, 6, 8].
The functionality of the autoregulation can be expressed as the autoregulatory index (ARI), with 0 being absent and 9 perfect cerebral autoregulation. This ARI has been shown to be lower in preeclampsia when compared with normotensive control subjects [14].
The principal posterior distribution of white matter alterations has been attributed to the sympathetic innervation of the cerebral vessel which accounts for the neurogenic response of cerebral autoregulation to hypertension.
This distribution has an anteroposterior gradient with a relative reduced innervation of the posterior cerebral arterial circulation. During acute elevation of blood pressure, posterior brain regions may be particularly susceptible to breakthrough of autoregulation.
It has been proposed that the fluid accumulation often observed during pregnancy, particularly in the third trimester, may accentuate the increase in development of vascular endothelial permeability in the brain. An increase in permeability can promote under hypertension [8].
Cortical blindness is a rare and dramatic complication of preeclampsia. Petechial hemorrhages and focal cerebral edema in the occipital cortex secondary to ischemic injury and vasogenic edema causing increased capillary permeability are considered to be important factors for the pathogenesis of cortical blindness in preeclampsia [15].
PRES has been less commonly described in the setting of autoimmune disease. The distinctive role of autoimmune disease is often clouded by concurrent hypertension, renal disease, and the use of immunosuppressants [10]. In autoimmune disorders, PRES is in part caused by endothelial dysfunction due to an inflammatory cytokine response that leads to autoimmune-mediated disruption of the endothelial aquaporin 4 water channels [10].
It is known that a woman who develops preeclampsia has a higher risk of developing cardiovascular disease later in life, as hypertension, ischemic, and hemorrhagic stroke. This condition leads formerly preeclamptic women to have cerebral white matter lesions more often and more severely than control women with normotensive pregnancies. Preeclampsia appears to be an independent risk factors for white matter lesions, and this was predominant in women with early-onset preeclampsia [16].
Another study assessed that an episode of PRES may play an additional role in increased prevalence of cerebral white matter lesions, visible in frontal lobes rather than in occipital lobes. Severe vasogenic edema has been suggested to potentially progress to such an extent that regional perfusion decreases, leading to areas of cytotoxic edema, infarction, and development of white matter lesions in the long term. In the same study, the authors reported that white matter lesions are more common in women with prior pregnancies complicated by preeclampsia or eclampsia compared with parous women in a control group [16].
Other two pathologic conditions are reported in pregnancy-related vascular encephalopathy: HELLP (hemolysis-elevated liver enzymes-low platelets) syndrome and RCVS (reversible cerebral vasoconstriction syndrome) [18]. The first is associated with strong epigastralgia, hepatic failure, disseminated intravascular coagulation, and intracerebral hemorrhage. The syndrome is caused by low placental gene expression of nitric oxide. Generally, MR imaging findings of HELLP syndrome may overlap with those of PRES (cerebral edema), with prevalent involvement of basal ganglia and brain stem [19]. Lacunar lesions are frequent [18].
Typical symptom of RCVS is a “thunderclap” headache due to multifocal or segmental cerebrovascular spasm, visible on MR angiography, which resolved within 12 weeks. Parenchymal lesions are frequently located in areas of the anterior circulation [18].
15.2.2 Ischemic Stroke, Venous Thrombosis, and Cerebral Hemorrhages
There is an increased risk of strokes in pregnancy and puerperium. Intracranial hemorrhage is secondary to hypertensive disorders of pregnancy with smaller proportions related to aneurysm and arteriovenous malformation rupture. A small but important contributor is cortical venous thrombosis. Presentation is usually with headaches or seizures, with or without focal deficits [20].
Pregnancy and the puerperium are considered hypercoagulable states. The risk of both ischemic infarction and intracranial hemorrhage increased with age and is high in the peripartum period and puerperium, but not during the pregnancy [21].
Cerebral venous thrombosis may occur anytime during the course of pregnancy and the puerperium, but the risk is high during the first 2 weeks of the puerperium. Venous sinus hyperdensity on CT acquisition and loss of venous flow-void artifact on MR images are typical findings (Fig. 15.4a–e). Venous infarcts do not represent the arterial territories and are often associated with hemorrhage at the gray-white matter interface [22].
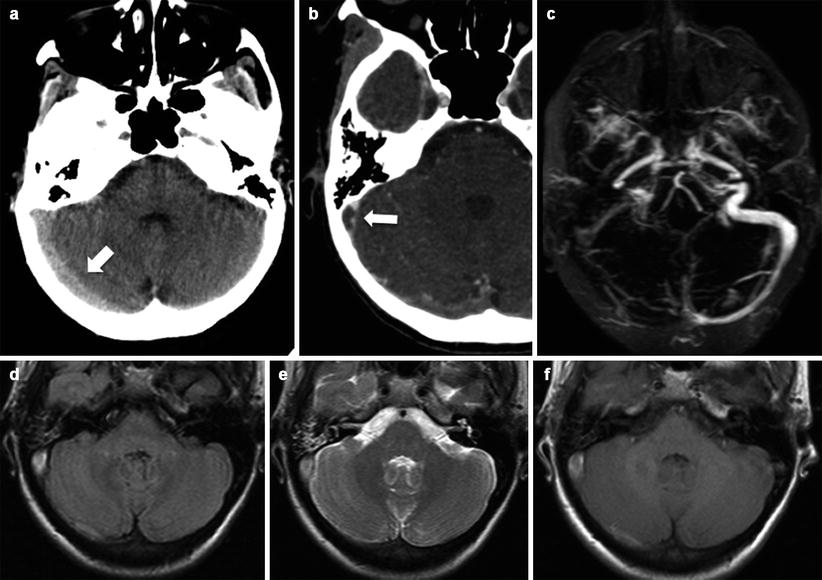

Fig. 15.4
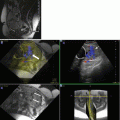
Venous thrombosis in a young woman on the 3rd month of pregnancy. Hyperdensity of transverse and sigmoid sinus are evident (a, arrow); loss of enhancement into the sinus after contrast injection (delta sign, b, arrow). MR investigation confirms venous thrombosis in contrastographic MR angiography study (c) and in FLAIR (d), T2- (e) and T1-weighted (f) images where the venous structures show high signal
Subarachnoid hemorrhage (SAH) is the third most important cause of maternal mortality during pregnancy in the United States. Maternal mortality due to rupture of an intracranial aneurysm ranges from 13 to 35 % [23].
The frequency of aneurysmal SAH during pregnancy increased with advancing gestational age, with more than 50 % of intracranial aneurysm ruptures occurring in the third trimester [24].
This condition is related to the gravid state: increases in blood volume and blood pressure and hormone’s increase. It is reported intracranial aneurysm formation, enlargement, and rupture during pregnancy [25]. The common site of intracranial aneurysm is the internal carotid artery [24].
MR angiography represents a screening technique able to discover intracranial vascular lesions [23].
Vascular malformations such as arteriovenous malformations, moyamoya disease, and cavernous malformations undergo morphological changes under the influence of female hormones and increase of blood levels of vascular growth factors that lead to an increase of incidence of bleeding [26].
15.3 Tumors
The frequencies of brain tumors appear to be similar for pregnant and nonpregnant women [27], whereas pregnancy seems to be involved in growth of some intracranial tumors.
Many studies report that pregnancy is associated with meningioma growth, due to the common expression of progesterone and estrogen receptor [28]. This was first recognized in the 1930s and documented in multiple reports of women with meningiomas who had symptom onset during pregnancy and remission during the postpartum period [29]. Other intracranial tumors that show dimensional increases during pregnancy are hemangioblastoma and vestibular schwannoma.
Large cohort studies show pregnancy by itself does not influence the risk of developing a glioma [30].
However, the role of pregnancy about the growth of gliomas is debated. Some authors reported that pregnancy has no significant effect on the incidence or behavior of glioma [21]; others assessed that the pregnancy may influence glioma behavior and the glioma may affect the course of pregnancy [30].
Other authors referred that in pregnant women with no clinical signs of glioma progression, detailed neuroimaging may well show an increase in tumor volume [30].
These phenomena are probably correlated with increase of blood volume due to hormonal increase that leads to exacerbated peritumoral edema and increase in mass effect. This mechanism could explain a return of tumor mass to prepregnancy volumes following delivery [30].
Two small retrospective case series described a potential impact of pregnancy on gliomal growth and transformation to higher-grade tumors [31, 32]. Similar results are reported about meningiomas [33]. The increased growth rate was associated with a higher frequency of seizures, and it is linked to the hormones and growth factors increase.
Several theories have been proposed to explain the progression of brain tumors during pregnancy. Hormonal changes and increases in the levels of growth factors and angiogenic factors during pregnancy influence the rate of growth of brain tumors [34]. Increased levels of vascular endothelial growth factor (VEGF) and placental growth factor are well-established angiogenic factors in GBM [35].
In a recent study, the authors reported that grade I gliomas remained stable during and after pregnancy. The different tumor biologies, characterized by minimal tumor infiltration and absence of angiogenesis, could explain the absence of tumor progression [36].
The link between brain tumors and hormones is known.
Tumor growth for specific tumors was found to depend on the stage of the pregnancy with meningiomas in the third trimester and gliomas in the first trimester [29]. The appearance of de novo high-grade gliomas in healthy pregnant women occurred mostly in the late second and third trimester [30].
Meningiomas more frequently express progesterone and glucocorticoid receptors; higher levels of progesterone receptors have been observed to be higher in GBMs as compared to anaplastic and low-grade astrocytomas [29].
15.4 Pituitary Disorders
Pituitary apoplexy is extremely rare during pregnancy and has been reported in only a few cases in the literature [37].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree