Fig. 2.1
Transvaginal sonography showing a pathologically thickened endometrial cavity (line) secondary to the presence of an endometrial carcinoma
If, because of clinical data or ultrasound findings, the presence of an endometrial carcinoma is suspected, histological evaluation is mandatory. To date, endometrial biopsy represents the most widely used technique for assessing the presence of EC and has largely replaced dilatation and curettage because of its lower cost, little or no need of anesthesia, and less traumatism. However, endometrial biopsy shows only a fair accuracy in assessing tumor type and grade: for example, up to 30 % of grade 1 tumors at endometrial biopsy will be upgraded to grade 2 or 3 on hysterectomy specimen.
2.3 Pathological Findings
The large majority of endometrial neoplasms arises from the endometrial glandular epithelium and is represented by adenocarcinomas of endometrioid type (75–85 %) (Fig. 2.2a) [8]. Endometrioid-type EC usually develops in the setting of a hypertrophic endometrium and often show a polypoid appearance; these lesions may be further subdivided into four subtypes according to their prevalent histologic aspects (with squamous differentiation and villoglandular, secretory, or ciliated cells). Moreover, every subtype can show three different grades of differentiation, from grade 1, well differentiated neoplasms, to grade 3, poorly or undifferentiated neoplasms [8]. Grade 1 endometrioid EC are constituted by well-formed glands, with no more than 5 % of solid non-squamous areas, whereas solid non-squamous areas represent 6–50 % of the mass in grade 2 neoplasms and more than 50 % of it in grade 3 ones [2, 14].
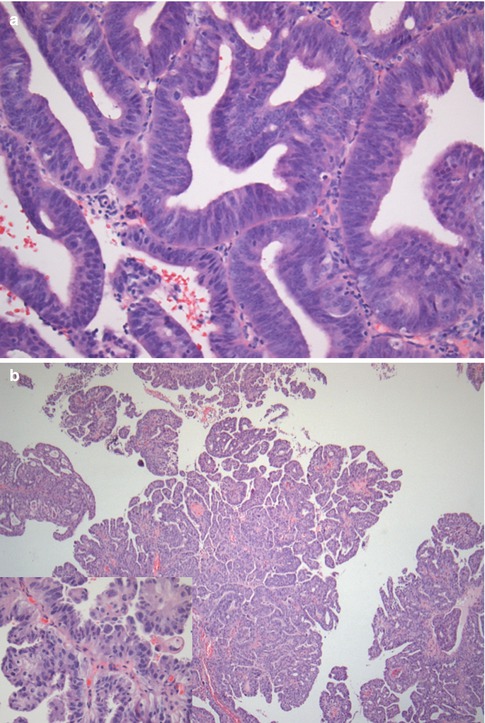
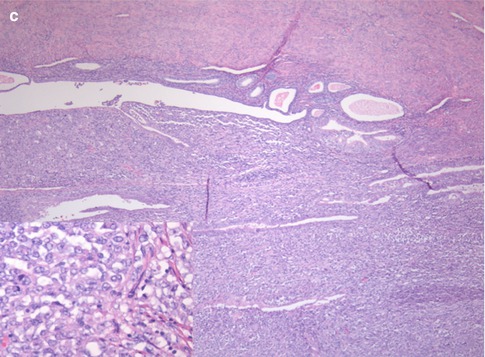


Fig. 2.2
(a–c) Different pathological appearances of endometrial cancer. G1 endometrioid-type endometrial carcinoma (a) is characterized by an overall conservation of endometrial glandular appearance with the presence of nuclear atypia, nuclear overlays, and an increased number of mitosis; the stroma is markedly reduced and atypical glands are densely packed. Serous-type endometrial carcinoma (b) shows a papillary architecture with more evident nuclear atypia (box). Endometrial stroma sarcoma (c) is characterized by glandular rarefaction (lower portion), in comparison with the physiological endometrium (upper portion), secondary to an abnormal expansion of endometrial stroma. At high magnification (box) the atypical stromal proliferation is clearly appreciable
Another 20–25 % of endometrial neoplasms are constituted by endometrial adenocarcinomas that are grouped together under the “non-endometrioid” diction (Fig. 2.2b) that comprises serous carcinomas, clear-cell carcinomas, mixed carcinomas, squamous-cell carcinomas, transitional-cell carcinomas, small-cell carcinomas, and undifferentiated carcinomas. All these neoplasms have a highly aggressive behavior, which makes unnecessary the definition of a grade; in particular, serous carcinoma is the most aggressive subtype of endometrial carcinoma and shows prognosis similar to ovarian carcinoma.
In a small percentage of cases, the endometrium may also be involved by tumors of stromal origin (Fig. 2.2c), which include endometrial sarcoma, mixed Müllerian tumor, and leiomyosarcoma, that are characterized by a highly aggressive behavior and by an extremely poor prognosis [8].
It is important to notice that mixed epithelial-stromal tumors exist and that they may lead to some errors in preoperative histological evaluation because only one of the two components might be sampled during endometrial biopsy.
2.4 Tumor Spread
The most common way of diffusion of endometrial carcinoma is direct infiltration of adjacent structures. In particular, EC, which arises within the endometrium, usually begins its spread by infiltrating the superficial myometrium (i.e., the junctional zone). The neoplasm may then advance infiltrating deeper myometrial portions, usually by means of a wide infiltrating front. In advanced stages, EC may also reach the serosa and extend to peritoneal cavity and to adjacent organs. A less common way of direct spread of EC involves cervical epithelium and cervical stroma with possible extension to vaginal walls [15, 16].
The second most common way of diffusion of EC involves the lymphatic pathways that lead to the parametrial, paracervical, and obturator lymph nodes, in case of tumors of the middle and lower uterine portions, and to the common iliac and para-aortic lymph nodes, in case of neoplasms involving the uterine fundus and tubaric angles [15–17]. Inguinal nodes may also be involved by means of a spread along the round ligament following adnexal or pelvic sidewall tumor involvement. The probability of lymphatic spread is minimal for neoplasms confined to the inner myometrial portion and increases parallel to the depth of myometrial infiltration [18, 19]. Moreover, the presence of cervical stromal infiltration and the histologic diagnosis of non-endometrioid-type EC are also associated with a significantly increased risk of nodal metastases.
Tumor extension to the uterine cornua and to Fallopian tubes provides another route of spread to adnexal structures and the peritoneal cavity; this kind of spread is obviously more common for tumors involving the uterine fundus and tubaric angles than for the ones involving the uterine body or neck [15, 16, 20].
Distant hematogenous metastases usually occur late in the biological history of EC, usually in locally advanced cases or in histological subtypes with poor prognosis, and mainly involve the lungs, liver, and bone marrow [8].
2.5 Tumor Staging
Since 1988, the FIGO (International Federation of Gynecology and Obstetrics) defines formal staging of endometrial carcinoma subdividing the neoplasms into four main stages (stage I, tumor confined to the corpus uteri; stage II, tumor involving cervical stroma; stage III, local/regional tumor spread; and stage IV, tumor invading other organs/structures) according to surgical and pathological data acquired after complete abdominal hysterectomy with bilateral salpingo-oophorectomy, pelvic and retroperitoneal lymphadenectomy, omentectomy or omental biopsies, and peritoneal washing [21, 22].
The 2009 revision (Table 2.1) of the 1988 FIGO classification of endometrial carcinoma is the one actually in use; it maintained the four main stages (I–IV) of differentiation, but simplified some further subdivisions. Stage I is actually subdivided into two substages (vs. 3 substages in the previous classification): Stage IA is characterized by myometrial infiltration <50 % of its thickness, and stage IB by myometrial infiltration >50 % of its thickness; no more difference is made between EC that exclusively involve the endometrium and EC that also infiltrate the inner half of the myometrium. Stage II actually has no substages (vs. 2 substages in the previous classification): cervical mucosa infiltration is no longer considered a staging parameter, whereas cervical stromal infiltration remains its unique characteristic. Stage III maintained three substages: stage IIIA is characterized by serosa and/or adnexal infiltration, stage IIIB by vaginal and/or parametrial involvement, and stage IIIC by nodal metastases. Two differences exist between the 1988 and 2009 classification of stage III: positive peritoneal cytology is no more considered a staging criteria (previously it was included in stage IIIA), and stage IIIC has been further subdivided into IIIC1 (pelvic nodal involvement) and IIIC2 (lomboaortic nodal involvement). No differences exist between the 1988 and 2006 classification of stage IV that maintained two substages: stage IVA, characterized by bladder and/or rectal infiltration, and stage IVB, showing distant metastases (including inguinal lymph nodes).
Table 2.1
The 2009 FIGO staging system of endometrial carcinoma and corresponding MRI features
Stage | FIGO description | MRI features |
|---|---|---|
IA | No/less than half myometrial invasion | Myometrium shows normal signal intensity profoundly to the neoplasm >50 % of its thickness |
IB | Equal to/more than half myometrial invasion | Myometrium shows normal signal intensity profoundly to the neoplasm <50 % of its thickness but serosa hypointense rim is preserved |
II | Cervical stroma infiltration | Interruption/signal intensity alteration of cervical stroma hypointense ring |
IIIA | Serosa infiltration and/or adnexal involvement | Interruption/signal intensity alteration of serosa hypointense rim and/or presence of an adnexal mass |
IIIB | Vaginal and/or parametrial involvement | Signal intensity alteration extending to the vagina and/or to the parametria |
IIIC1 | Pelvic lymph node metastases | Pathologically enlarged (>10 mm in minimum diameter) pelvic lymph nodes |
IIIC2 | Para-aortic lymph node metastases | Pathologically enlarged (>10 mm in minimum diameter) retroperitoneal lymph nodes |
IVA | Bladder and/or bowel mucosa infiltration | Loss of cleavage plans between the neoplasm and the bladder and/or the bowel walls that appear thickened |
IVB | Distant metastases (including inguinal lymph nodes) | Pathologically enlarged (>10 mm in minimum diameter) inguinal lymph nodes or other distant metastases (often not comprised in MRI scans) |
2.6 Prognosis
The prognosis of a patient affected by EC is overall better than for other gynecological malignancies, but it depends on the histological type, tumor grade, and tumor stage.
Non-endometrioid-type EC is associated with an overall poor prognosis; indeed, although representing only about 20 % of the cases, non-endometrioid EC account for more than 50 % of all recurrences and deaths for endometrial carcinoma [23]. On the other hand, endometrioid-type EC show an extremely variable prognosis, depending on the tumor grade and stage; for example, the prevalence of nodal metastases, which are correlated with a poor prognosis, is significantly higher in patients affected by G2–G3 endometrioid-type EC than in patients affected by G1 ones.
Besides tumor type and grade, the 5-year survival rates (5 years) of endometrial carcinoma are also strictly correlated with tumor stage at the time of diagnosis. Stage IA EC show a 5 years of about 89.6 %, stage IB ones of 77.6 %, and stage II of 70.2 %. The 5 year survival rates of EC significantly decrease for stage III neoplasms, to about 49.2 %; the presence of nodal metastases is associated with 5 years of 57 % for pelvic nodal involvement only (stage IIIC1) and 49 % for para-aortic nodal involvement (stage IIIC2) [24]. Stage IV EC is associated with an extremely poor prognosis, with a 5 years of 18.7 %.
The patient’s overall physical performance status and age are also strictly correlated with prognosis [25].
2.7 Treatment
The standard surgical treatment for endometrial carcinoma implies laparotomy and comprises complete hysterectomy with bilateral salpingo-oophorectomy, pelvic and retroperitoneal lymphadenectomy, omentectomy or omental biopsies, and peritoneal washing. Nowadays, however, the improvements in preoperatory staging techniques, namely, ultrasound (US), magnetic resonance (MR), and computed tomography (CT), and in surgical techniques have determined an increase in the request of less invasive treatment approaches. Currently, laparoscopy and robotic surgery approach are preferred over laparotomy in the large majority of cases, and a recent publication by the Gynecologic Oncology Group (GOG), the LAP2 study, has shown that laparoscopy provides equivalent results in terms of disease-free survival and overall survival, compared with laparotomy, with the advantage of shorter hospital stay, less use of painkillers, lower rate of complications, and improved quality of life [24]. Moreover, despite the surgical approach, nowadays the extent of surgery must also be modulated according to histological data and to preoperative staging findings; for example, lymphadenectomy, which is burdened by significant complication rates and postoperative morbidity, has shown no benefits in terms of survival and recurrence-free rates in patients affected by low-risk (grade 1, stage IA) EC [26–28], whereas it remains crucial for improving the prognosis in high-risk patients [29–31].
Adjuvant chemotherapy is usually administered to all patients affected by non-endometrioid-type EC or by endometrioid-type G2–G3 ones and to patients affected by FIGO stage II to IV neoplasms. Patients affected by surgically unresectable EC and patients showing absolute clinical contraindications to surgery may benefit from radiation therapy in order to reduce bleeding risk; however, maximal surgical debulking should be performed also in case of unresectable neoplasms in order to increase life expectancy. In case of hemorrhage, uterine artery embolization may be useful for reducing and/or stopping blood loss.
Therefore, in the era of individualized treatments, an accurate preoperative staging is crucial in order to tailor the best surgical approach for each patient. Many imaging modalities are actually available for locoregional staging of endometrial carcinoma, but magnetic resonance imaging must be considered the imaging modality of choice, thanks to its panoramicity, high tissue contrast resolution, and reproducibility [32–36].
2.8 The Role of MRI in Endometrial Carcinoma
MRI is not indicated for diagnosing endometrial carcinoma; indeed, although recent studies have demonstrated that apparent diffusion coefficient (ADC) values can reliably differentiate benign from malignant endometrial lesions [37, 38], hysteroscopy with endometrial biopsy remains the gold standard for this aim. On the other hand, nowadays MRI plays a well-defined role in the preoperative workup of patients with histologically proven EC [31, 39–43].
The main aims of preoperative MRI staging of endometrial carcinoma are the assessment of “T” stage (i.e., the quantification of the depth of myometrial infiltration, the identification of cervical stromal infiltration, and the assessment of extrauterine spread) and the definition of “N” stage (i.e., the identification of nodal metastases). MRI offers an overall high accuracy (81–92 %) [43, 44] in the definition of the “T” stage of endometrial carcinoma, which is significantly higher in comparison to CT, PET/CT, and transvaginal ultrasound [33–35, 45–48], but still lower than intraoperative pathological evaluation of frozen sections [49, 50]. Recent works report a high accuracy of MRI (89–97 %) [51, 52] also in the evaluation of the “N” stage of endometrial carcinoma. Such promising results, however, must be critically evaluated because of the influence of low pretest probability of nodal metastases in endometrial carcinoma (about 20 %). Indeed, the sensitivity of MRI in identifying nodal metastases, as reported in the same abovementioned studies, ranges from 55 to 67 %. However, despite the unsatisfactory sensitivity of MRI in directly recognizing nodal metastases, the MRI information about the “T” stage and, in particular, about the depth of myometrial infiltration and the presence of cervical stromal infiltration, in association with pathologic information about the tumor type and grade, enables to confidently predict the risk of nodal metastases, subdividing endometrial carcinoma into three groups (low, intermediate, and high risk) (Table 2.2) with consequent therapeutic implications [30, 36, 40].
Table 2.2
Risk stratification for the presence of nodal metastases and for recurrence of endometrial carcinoma (EC), according to MRI and pathology data
MRI stage | Histological type | Histological grade | |
|---|---|---|---|
Low-risk EC | T1a | Type 1 | G1–G2 |
Intermediate-risk EC | T1a | Type 1 | G3 |
Intermediate-risk EC | T1b | Type 1 | G1–G2 |
High-risk EC | T1b | Type 1 | G3 |
High-risk EC | T≥2 | Type 1 | G1–G2–G3 |
High-risk EC | Every T | Type 2 | G3 |
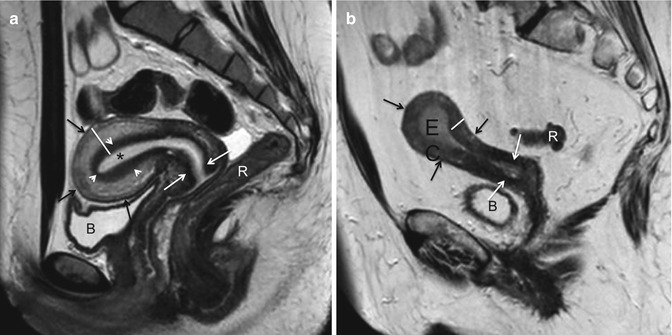
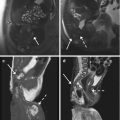
The secondary aims of preoperative MRI in EC are the evaluation of pelvic anatomy and the recognition of eventual pelvic comorbidities; the latter information, in particular, can be extremely helpful for a correct surgical planning. For example, the identification of coexistent large pelvic masses (Fig. 2.3) or of voluminous uterine fibromas might advise against a laparoscopic or transvaginal approach.
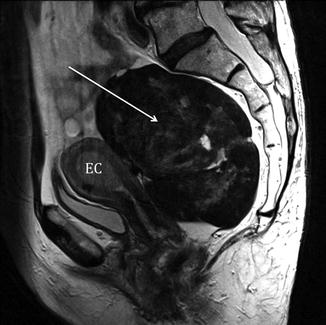

Fig. 2.3
Incidental MRI findings. Sagittal TSE T2-weighted image (TR/TE 2,400/76 ms) showing an endometrial carcinoma with polypoid appearance (EC). Dorsally to the uterus, in the presacral region, a large rounded T2-hypointense mass (arrow) is clearly recognizable. In this case, because of the coexistence of the pre sacral mass, laparotomy has been preferred to a laparoscopy. At pathology the presacral mass has been classified as a solitary fibrous tumor of the mesorectum
Nowadays, an extremely important means that enables to increase the quality of MRI staging of endometrial carcinoma and to improve diagnostic skills is the institution of radiological-surgical-pathological-oncological tumor boards in which each case can be preoperatively collegially discussed and postoperative feedbacks can be obtained.
2.9 Technical Requirements
MRI of the uterus must be performed on high field magnets (1.5 T or more) using multichannel (four or more channels) phased array body coils, in order to warrant an adequate signal-to-noise ratio. Recent studies have demonstrated that 3 T magnets do not increase MRI accuracy in staging endometrial carcinoma in comparison with 1.5 T ones [53, 54]. Moreover, the use of endorectal or endovaginal coils is not indicated because of their lower panoramicity in comparison with body coils [54].
The patient must fast at least 4–6 h before the examination, and 20 mg of hyoscine butylbromide should be intramuscularly administered 5 min before the patients enters the scanning room; alternatively, if hyoscine butylbromide is contraindicated, 5 mg of glucagon may be intravenously administered just before the beginning of the examination, but its effect might not last for the entire examination time.
An adequate bladder filling is essential in order to obtain high-quality images: in fact, a completely void bladder produces a bundling of the pelvic structures, thereby increasing the difficulties in recognizing the physiological cleavage planes, whereas an overfilled bladder stimulates bowel peristalsis, therefore increasing motion artifacts, and reduces the quality of T2-weighted and diffusion-weighted images. Intermediate bladder repletion may be obtained by suggesting the patient to void about 60 min before the examination and to hold urine from that time on.
The patient is usually positioned supine on the MR table with the arms lying down along her body, but prone position can be also considered in claustrophobic patients. MRI sequences for the study of the uterus can be acquired in free breathing, but the patient must be accurately instructed to maintain regular superficial breathing during the whole examination time, reducing the movements of the abdomen as much as possible.
MRI sequences are usually acquired using anteroposterior phase encoding, in case of sagittal images, and using laterolateral phase encoding in case of axial and coronal images. The placement of a presaturation bar over the anterior abdominal wall significantly increases image quality by reducing breathing-related motion artifacts. In order to further reduce abdominal wall motion artifacts, sagittal images may be acquired using a feet to head phase codification, with 100 % phase oversampling (50 % cranial and 50 % caudal to the region of interest).
2.10 MRI Protocol
Our suggested MRI protocol for the study of endometrial carcinoma is presented in Table 2.3 and is based on T2-weighted sequences, diffusion-weighted sequences, and pre- and post-contrast T1-weighted sequences.
Table 2.3
Suggested MRI protocol for the study of endometrial carcinoma
Pulse sequence | Orientation | Region | TR/TE (ms) | Fov (mm) | Voxel size (mm) |
|---|---|---|---|---|---|
TSE T1 weighted | Axial | Abdomen | 730/10 | 350 × 260 | 5 × 1 × 1 |
FS TSE T2 weighted | Axial | Abdomen | 7,700/83 | 400 × 400 | 5 × 1 × 1 |
TSE T2 weighted | Sagittal, axial, coronal | Uterus | 2,400/76 | 230 × 230 | 4 × 0.5 × 0.7 |
EPI diffusion weighted (b = 0, 500, 1,000) | Sagittal, axial, coronal | Uterus | 3,100/98 | 250 × 250 | 5 × 2 × 2 |
CE TSE T1 weighted | Sagittal, axial, coronal | Uterus | 660/9.5 | 250 × 250 | 4 × 0.8 × 0.9 |
First of all, large field of view (350 × 350 mm) TSE T1-weighted images must be acquired, according to the axial plane, from femoral heads to renal hila; these images play a fundamental role in the identification of enlarged lymph nodes, in the evaluation of the interface between the uterus and periuterine fat, and as a roadmap for the precise positioning of subsequent sequences. On the other hand, native T1-weighted images are not able to differentiate the different uterine structures, and also endometrial carcinoma, because of their low intrinsic tissue contrast resolution. Optionally or in addition to the abovementioned TSE T1-weighted images, the acquisition of large field of view axial fat-saturated T2-weighted RARE (rapid acquisition with relaxation enhancement) images, even from femoral heads to renal hila, may increase the accuracy of MRI in identifying retroperitoneal enlarged lymph nodes; in fact, the long repetition time of these sequences generates a “flow void” artifact into the lumen of blood vessels that therefore appear hypointense and thus can be easily differentiated from lymph nodes, which show an intermediate signal intensity/hyperintensity on these sequences and show up within the hypointense “saturated” abdominal fat.
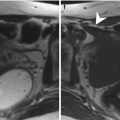
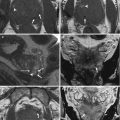
All subsequent imaging sequences must be acquired using small fields of view (about 250 × 250 mm, depending on the patient’s size) and large matrix, in order to warrant a high spatial resolution, according to the three orthogonal planes (parasagittal, para-coronal, and para-axial) of longest endometrial axis (Fig. 2.4a, b).
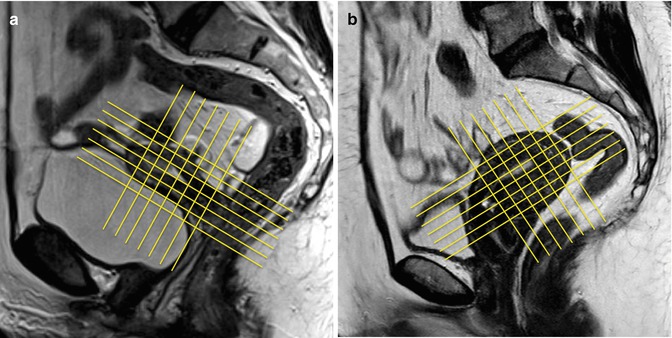

Fig. 2.4
(a, b) MR scanning plans for the study of the endometrium. The yellow lines on these sagittal TSE T2-weighted images (TR/TE 2,400/76 ms) show the correct orientation of uterine scanning plans. In order to avoid possible staging errors, MRI scanning planes must be always orientated orthogonal to the endometrial longer axis, on the sagittal, axial, and coronal planes, despite the uterine position, like in these two cases representing both an anteroflexed (a) and a retroflexed (b) uterus
TSE T2-weighted images play a central role in staging endometrial carcinoma because of their high soft tissue contrast resolution in association with their high spatial resolution that warrants an excellent anatomical detail. On TSE T2-weighted images, the physiological uterine wall stratification (the endometrium, the myometrium with the junctional zone, and the serosa), the cervical structures (mucosa and stroma), the vagina, and adjacent organs and structures are clearly recognizable, and it is possible to carefully differentiate endometrial carcinoma from the abovementioned structures. Therefore, TSE T2-weighted images are crucial for local staging of EC.
Contrast-enhanced (CE) TSE T1-weighted images must be acquired after i.v. administration of 0.05–0.1 mmol/Kg of gadolinium chelates. They are extremely useful for local staging of neoplasms that appear isointense in comparison to adjacent structures on T2-weighted images and in elderly women, in which the disappearance of junctional zone reduces the signal-to-noise ratio between the neoplasm and the myometrium.
Diffusion-weighted images are collecting increasing consensuses for MRI staging of endometrial carcinoma; indeed, technical developments have made it possible to obtain high-b-value diffusion-weighted images with an adequate spatial resolution in short acquisition times. Diffusion-weighted images are extremely useful for identifying small endometrial carcinomas, which may be often barely recognizable on conventional MRI sequences, and for quantifying the depth of myometrial infiltration; indeed, their high sensitivity in identifying hypercellular tissues is useful for the recognition of small neoplastic spicule within the myometrial layer.
2.11 Normal Uterine Anatomy
As mentioned before, uterine anatomy is best depicted on TSE T2-weighted images (Fig. 2.5a, b). In premenopausal women (Fig. 2.5a), the uterine wall shows four well-defined different layers on T2-weighted MR images that reduce to three with progressive aging (Fig. 2.5b). Going from the center to the periphery, the innermost layer is constituted by the endometrium, which shows high signal intensity on T2-weighted images and presents a physiologic thickness variable from 1 to 7 mm, according to the patient’s age and phase of the menstrual cycle. The following layer is the so-called junctional zone, or intermediate layer. The junctional zone appears hypointense on T2-weighted images and is constituted by the innermost portion of the myometrium; it has not a precise correspondence on pathological specimens, and its MRI differentiation from the outer portion of the myometrium is due to its relatively lower water content. In postmenopausal age the junctional zone shows a progressive increase in its water content, and, consequently, its signal intensity on T2-weighted images becomes substantially isointense to the outer portion of the myometrium [42]. The outer layer of the myometrium shows intermediate signal intensity on T2-weighted images, both on pre- and postmenopausal women. The overall myometrial thickness is usually between 14 and 21 mm and shows a progressive reduction with patient’s aging; it is crucial to remember that the myometrium becomes progressively thinner approaching the tubaric angles. The outermost layer of the uterine wall that can sometimes be barely recognizable appears as a thin T2-hypointense line and is constituted by the uterine serosa. Cervical structures are also well depicted on T2-weighted images. Cervical mucosa shows the same signal intensity of the endometrium, but appears thinner, whereas cervical stroma appears markedly hypointense on T2-weighted images.